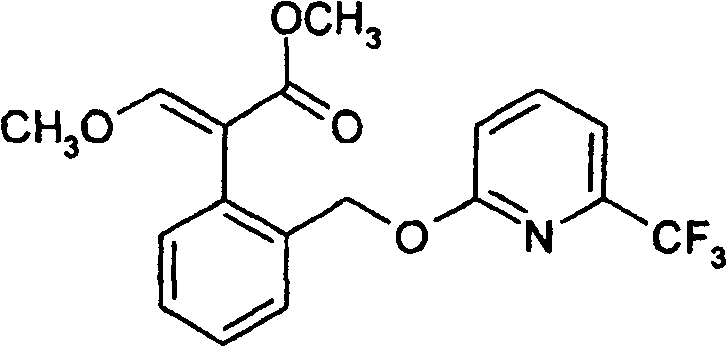
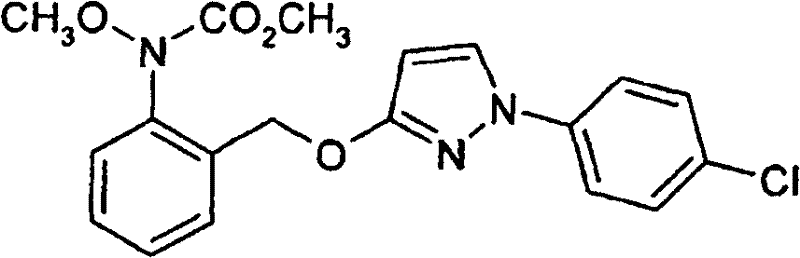
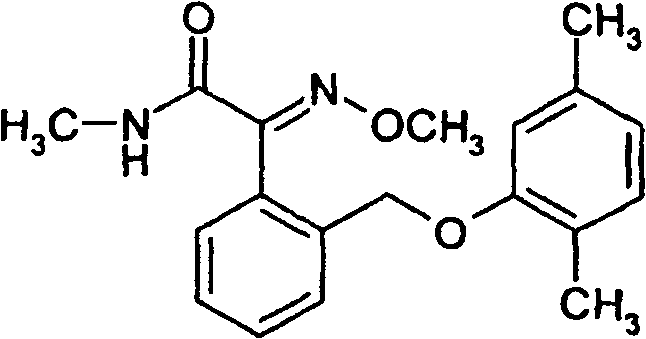
Insecticides based on selected neonicotinoids and methoxyacrylates
A pest and clothianidin technology, applied in the direction of insecticides, biocides, fungicides, etc., can solve the problems of not meeting the high requirements of insecticides and low application rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0342] Myzus persicae test
[0343] Solvent: 7 parts by weight of dimethylformamide
[0344] Emulsifier: 2 parts by weight of alkyl aryl polyglycol ether
[0345] To prepare a suitable active compound preparation, 1 part by weight of the active compound is mixed with the stated amount of solvent and emulsifier, and the concentrate is diluted with water containing the emulsifier to the desired concentration.
[0346] Cabbage (Brassica oleracea) leaves severely infested by the green peach aphid (Myzus persicae) are immersed in the active compound preparation of the desired concentration for treatment.
[0347] After the required time, the mortality rate is determined in %. 100% means that all aphids have been killed; 0% means that no aphids have been killed.
[0348] Substitute the determined mortality rate into Colby's formula.
[0349] In this test, for example, the following active compound combinations of the present application showed synergistic activity relative to the active compou...
Embodiment B
[0361] The larvae test of horseradish beetle
[0362] Solvent: 7 parts by weight of dimethylformamide
[0363] Emulsifier: 2 parts by weight of alkyl aryl polyglycol ether
[0364] To prepare a suitable active compound preparation, 1 part by weight of the active compound is mixed with the stated amount of solvent and emulsifier, and the concentrate is diluted with water containing the emulsifier to the desired concentration.
[0365] Cabbage (Brassica oleracea) leaves are immersed in the active compound preparation of the desired concentration for treatment, and horseradish beetle (Phaedon cochleariae) larvae are inserted while the leaves are still moist.
[0366] After the required time, the mortality rate is determined in %. 100% means that all beetle larvae have been killed; 0% means that no beetle larvae have been killed. Substitute the determined mortality rate into the Colby formula (see Table 1).
[0367] In this test, the following active compound combinations of the present ap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com