A pharmaceutical container for the quantitative release of a single dose of t3 and t4 thyroid hormone solution for oral administration
A thyroid hormone, single-dose technology, used in pharmaceutical formulations, drug combinations, drug delivery, etc., can solve problems such as not being recommended, thyroid hormones not known, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0039] Preparation of Glycerol-Ethanol Solution of Levothyroxine Sodium (T4)
[0040] Composition and quantity for the preparation of 25 L:
[0041] Levothyroxine Sodium (T4): 2.625g
[0042] Glycerol (85%): 21.525kg
[0043] Ethanol (96%): 6.100kg
[0044]In a 10 L stainless steel vessel fitted with a paddle stirrer and lid, add 90% ethanol (5.49 L) and add T4 with stirring; stir slowly while maintaining nitrogen flow until complete dissolution. Glycerol (21.525 kg) was poured into a 25 L turbo emulsifier (Olsa-Italy) and an ethanol solution containing the T4 solution was added. Rinse the 10 L stainless steel vessel with the remaining ethanol (0.61 L) and pour it into a 25 L turbo emulsifier. Stirring was continued at low speed for 15 minutes under a nitrogen environment and protected from light.
example 3
[0060] Preparation of 1.0 ml (1.3 ml fill) nominal, neutral LDPE / EVA single-dose plastic containers with screw caps containing levothyroxine sodium (T4) in glycerol-ethanol solution.
[0061] a) Fabrication of single-use containers
[0062] Composition of materials, amounts and relative percentages used for preparation:
[0063] Low-density polyethylene (LDPE): 25.0kg, 50%
[0064] Ethylene-vinyl acetate copolymer (EVA): 25.0kg, 50%
[0065] The product contains 5 single-dose strips of 1.0 ml with screw caps.
[0066] 5 single-dose strips and 5 cap strips were produced by injection molding using two different molds and then assembled with semi-automatic equipment.
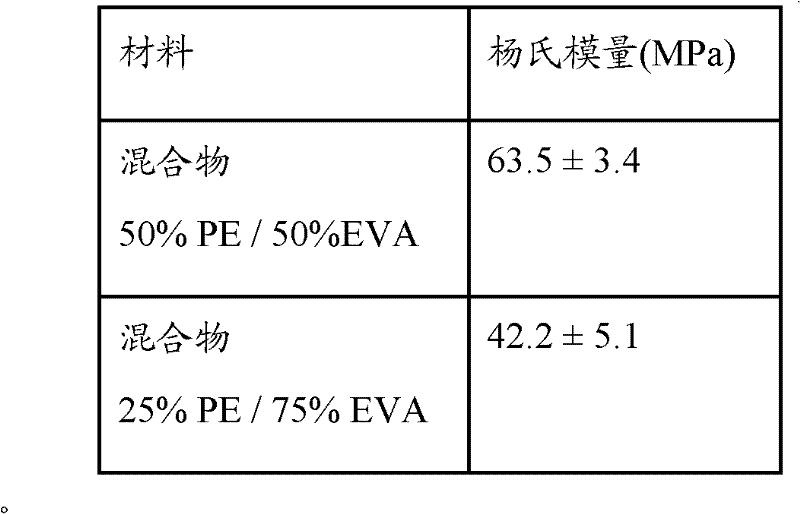
[0067] The characterization of the injection molded products is carried out by determining the Young's modulus: the UNI-EN-ISO 527-1 reference standard is used to determine the sample size and the pulling is carried out using a pulling speed of 5 mm / min.
[0068] 50LDPE / 50EVA mixture
63.5MPa
...
example 4
[0077] Preparation of 1.0 ml (1.3 ml fill) nominal, neutral LDPE / EVA single-dose plastic containers with screw caps containing levothyroxine sodium (T4) in glycerol-ethanol solution.
[0078] a) Fabrication of single-use containers
[0079] Composition of materials, amounts and relative percentages used for preparation:
[0080] Low-density polyethylene (LDPE): 12.5kg, 25%
[0081] Ethylene-vinyl acetate copolymer (EVA): 37.5kg, 75%
[0082] The product contains 5 single-dose strips of 1.0 ml with screw caps.
[0083] 5 single-dose strips and 5 cap strips were produced by injection molding using two different molds and then assembled with semi-automatic equipment.
[0084] The characterization of the injection molded products is carried out by determining the Young's modulus: the UNI-EN-ISO 527-1 reference standard is used to determine the sample size and the pulling is carried out using a pulling speed of 5 mm / min.
[0085] 25LDPE / 75EVA mixture
42.2 MPa
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com