Application of compound 4-o-methyl-benzenesulfonyl-2(3H)-benzoxazolone and pharmaceutically acceptable salt thereof in preparing non-steroidal anti-inflammatory and analgesic medicines
A benzenesulfonyloxybenzoxazolone, non-steroidal anti-inflammatory technology, applied in the field of new compound uses, can solve the problem of no relevant reports on the pharmacological effect of anti-inflammatory and analgesic activity, and achieve the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
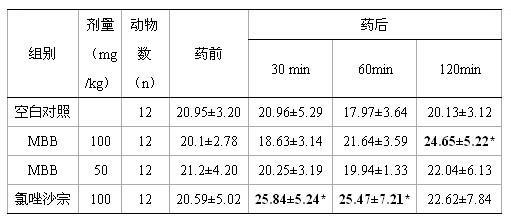
[0014] 1. Effect of compound MBB on pain threshold of hot plate in mice
[0015] 1.1 Experimental steps:
[0016] Kunming mice were placed in a water bath with a water temperature of 55°C, and the mice kicked or licked due to heat stimulation.
[0017] Response to rear foot or limb movement, which is used as an indicator of pain response. Select 48 female mice (choose the mouse that licks the feet within 5 seconds and 30 seconds on the hot plate, and the unqualified ones are eliminated), and they are randomly divided into 4 groups, 12 in each group, which are respectively physiological saline control group, the invention The compound MBB administration group (suspension prepared with 0.5% sodium carboxymethylcellulose) and the positive control group (chlorzoxazone) were intragastrically administered to rats (see Table 1 for intragastric administration dosage). Afterwards, the time from when the mouse was placed on the hot metal plate to when the pain response occurred was re...
Embodiment 2
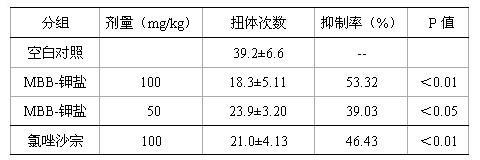
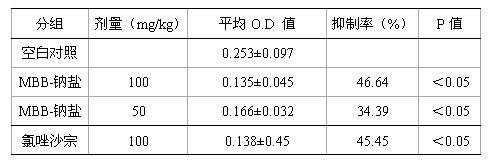
[0032] 1. The effect of compound MBB on the writhing response of mice induced by acetic acid
[0033] 1.1 Experimental steps:
[0034] Kunming strain mice, weighing 20±2g, were randomly divided into normal saline control group, MBB administration group of the compound of the present invention and positive control group (chlorzoxazone). Each group was intragastrically administered once a day (see Table 3 for intragastric administration dosage) for 3 consecutive days. One hour after the last administration, each mouse was intraperitoneally injected with 0.2ml of 0.6% acetic acid, and then observed the torsion caused by acetic acid within 15 minutes. Body times.
[0035] 1.2 Calculation and statistical analysis of experimental results (Table 3):
[0036] Inhibition rate (%) = (writhing times of blank control group - writhing times of drug administration group) / writhing times of blank control group × 100%.
[0037] Table 3 The effect of compound MBB on the number of times of ...
Embodiment 3
[0085] 1. Effect of compound MBB on carrageenan-induced toe swelling in rats
[0086] 1.1 Experimental steps:
[0087] Take 40 rats, half male and half female, weighing 160-180g, and randomly divide them into five groups according to body weight, 8 rats in each group, respectively: solvent control group (i.e. model group); MBB effect group (including three doses) and Positive control group (chlorzoxazone). Rats in each group were given 0.5% sodium carboxymethylcellulose, compound MBB and positive drug every day. The animals were gavaged once a day (see Table 9 for the gavage dose) for 3 consecutive days. One hour after the last administration, the diameter of the normal toe of the left hind limb of the rats in each group was accurately measured with a vernier caliper. Make a mark around the ankle joint of the hindlimb, and then subcutaneously inject 0.15ml of 1% carrageen suspension in the toes of the left hindlimb of the rat to cause inflammation. Subsequently, eve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com