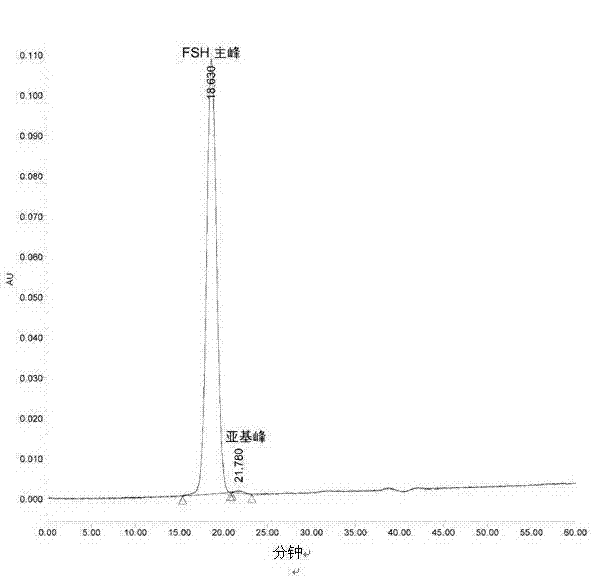
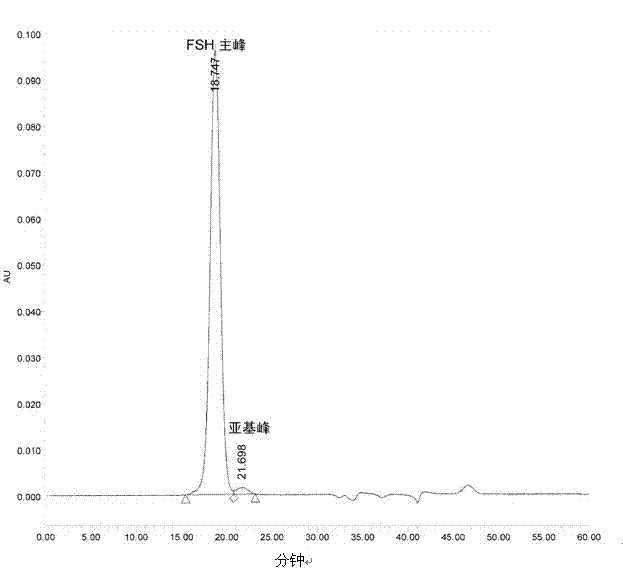
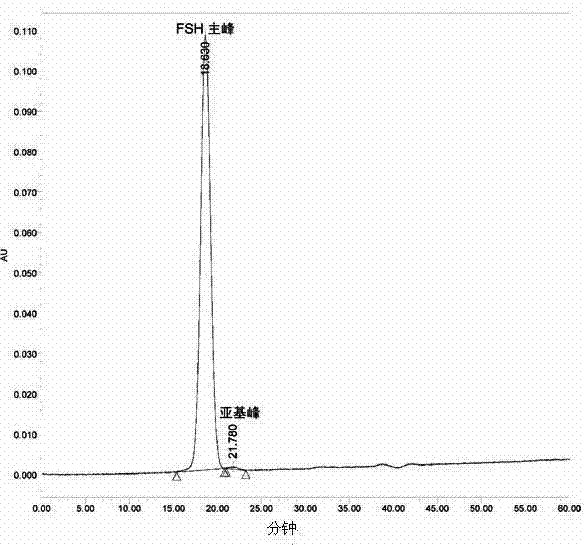
Composition of glycoprotein scarcely containing subunits
A composition and glycoprotein technology, applied in the field of protein drug purification, can solve the problems of inactivation of glycoproteins, easy inactivation of glycoproteins, not easy to transport, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] The present invention also provides a method for preparing a glycoprotein composition, the method comprising the steps of:
[0063] (a) raising the temperature of the pre-frozen glycoprotein-containing aqueous solution to the sublimation drying temperature at a heating rate of 0.05-5° C. / minute under vacuum, and maintaining it for 1-30 hours; and
[0064] (b) Elevate the temperature to the analytical drying temperature at a rate of 0.05-5° C. / min, and maintain it for 1-20 hours to obtain a composition containing glycoprotein, and the composition preferably contains a freeze-dried powder of glycoprotein. Said composition is a composition of glycoproteins whose subunit content is not more than 10 wt%.
[0065] The advantages of the present invention are:
[0066] The freeze-drying method provided can reduce or hardly generate subunit degradation, thereby maintaining the activity of the glycoprotein, reducing the generation of impurities, and obtaining a glycoprotein almo...
Embodiment 1
[0078] Freeze-drying of FSH
[0079] Prepare 50mL of 0.01M sodium dihydrogen phosphate solution (adjust the pH to about 6.5 with NaOH), add 2g of lactose, stir to dissolve, and filter with a 0.22μm filter. Take 10 mL of the filtrate, add 10.0 mg of the above-mentioned high-purity FSH (purchased from Shanghai Tianwei Biopharmaceutical Co., Ltd.) (biological potency is 8817 international units / mg), after complete dissolution, the eutectic point of this solution is about -2 ℃, put it into a lyophilizer (purchased from Virtis), and freeze-dry according to the following procedure:
[0080] 1. Prefreeze the shelf at -40°C for 3 hours;
[0081] 2. Cold trap to -45°C;
[0082] 3. Start the vacuum pump;
[0083] 4. The shelf temperature is raised from -40°C to -10°C at a rate of 0.125°C / min, and the shelf temperature reaches -10°C and maintained for 15 hours;
[0084] 5. The shelf temperature is raised from -10°C to +20°C at a rate of 0.3°C / min, and the shelf temperature reaches +2...
Embodiment 2
[0101] Preparation of FSH freeze-dried injection
[0102] A typical example of the production of 10,000 vials of FSH freeze-dried injections, each containing 75IU FSH, is as follows:
[0103] A calculated amount (in units of biological potency) of FSH essence was dissolved in 50 mL of pyrogen-free water for injection, if necessary, adjusted to pH 6.5±0.2 with HCl or NaOH, and then sterile-filtered with a 0.22 μm filter .
[0104] Dissolve 100 g of lactose in 2 L of pyrogen-free water for injection, adjust the pH to 6.5 ± 0.2 with HCl or NaOH if necessary, and perform sterile filtration with a 0.22 μm filter. Then add it to the above FSH solution, dilute to 7.5L with pyrogen-free water for injection, and mix well. The eutectic point of this solution is about -2°C.
[0105] The above solution was divided into ampoules, 0.75mL per bottle, and freeze-dried according to the following procedure:
[0106] 1. Prefreeze the shelf at -40°C for 3 hours;
[0107] 2. Cold trap to -45°...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com