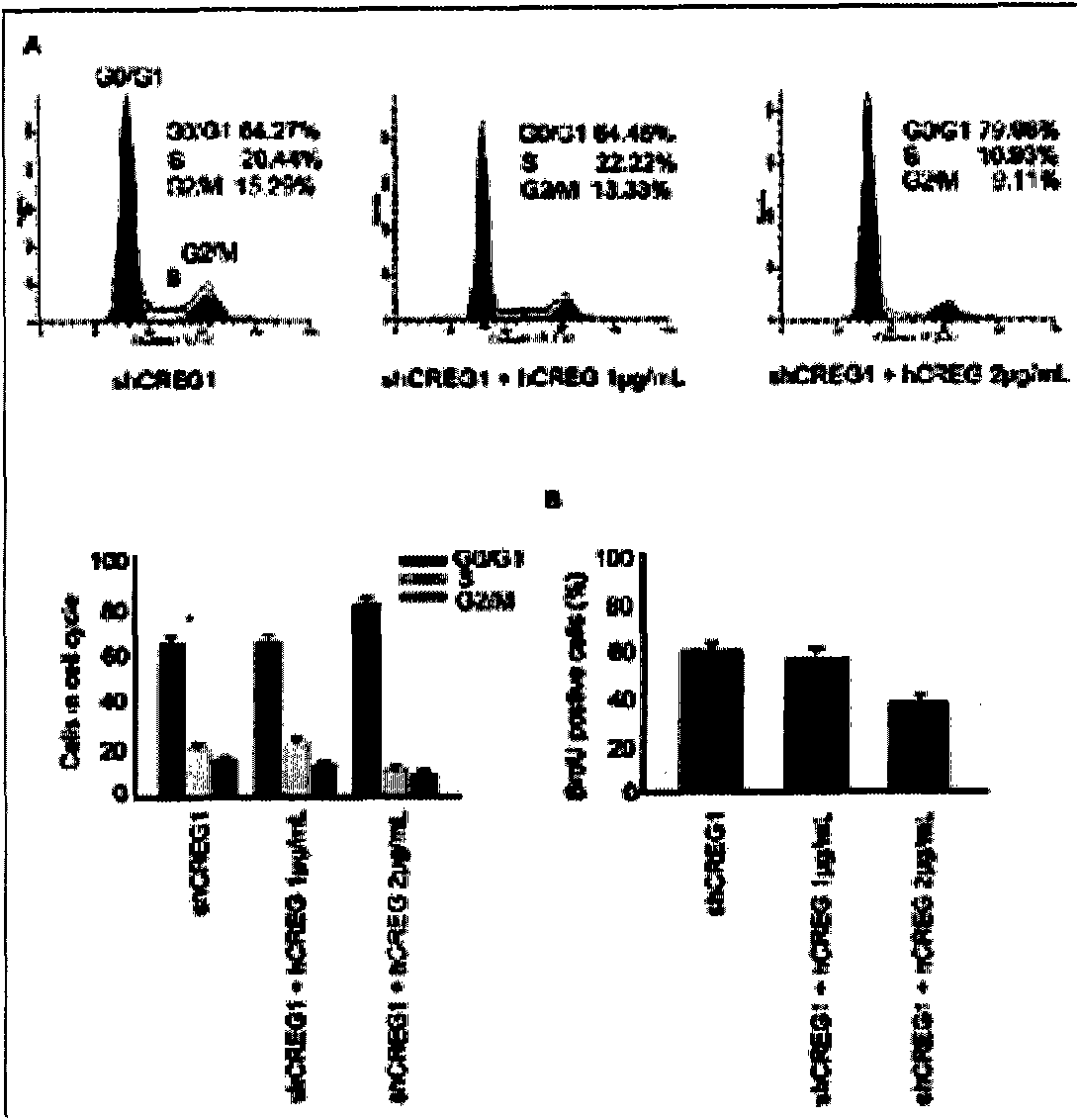
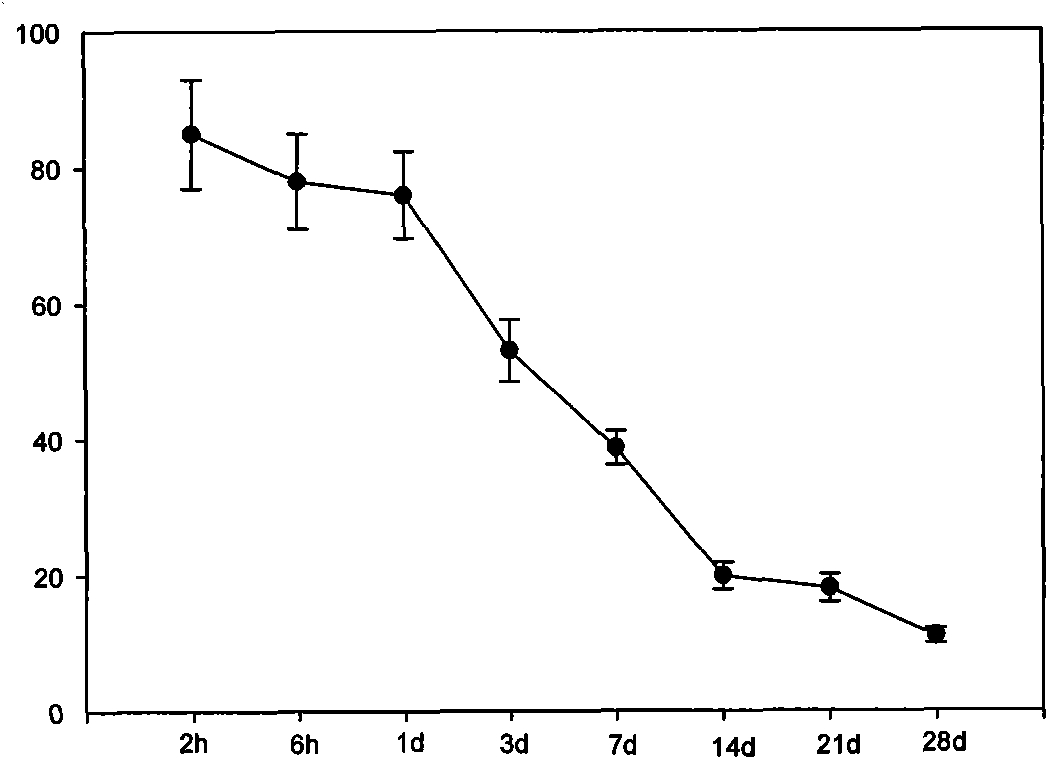
Coronary stent containing recombinant human cellular repressor of E1 A-stimulated genes (hCREG) glycoprotein and preparation method for coronary stent
A glycoprotein, coronary artery technology, applied in the field of coronary stents containing recombinant hCREG glycoprotein and its preparation, can solve problems such as low expression level, and achieve the effects of inhibiting cell proliferation, promoting healing, and promoting re-endothelialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of recombinant hCREG glycoprotein and its coronary stent
[0050] 1. Preparation of Nanoporous Recombinant hCREG Glycoprotein Elution Scaffold
[0051] 1.1 Preparation of recombinant hCREG glycoprotein
[0052] 1.1.1 Construction of recombinant gene vector Since 1999, the mRNA differential display technology was used to successfully clone the human CREG gene from the human internal thoracic artery VSMCs cultured in vitro for the first time, and the hCREG open reading frame with a stop codon mutation was amplified by RT-PCR technology Frame, the hCREG RT-PCR primers of the stop codon mutation are as follows: the upstream primer is 5'-aa ggatccatggccgggctatcccgc-3' (SEQ ID NO.1), and the downstream primer is: 5'-gc gaattcGcactgaactgtgacatttaatattcttctgg-3' (SEQ ID NO.2 ), where the capital letter represents the stop codon for the C / G mutation.
[0053] The recombinant human CREG protein expression vector pcDNA3.1-his / myc-hCREG with his tag protein ...
Embodiment 2
[0064] Embodiment 2 Preparation of drug-protein combined scaffold
[0065] The surface of the coronary stent in this embodiment is uniformly distributed with recombinant hCREG glycoprotein and rapamycin. The drug rapamycin is distributed on the outer layer of the stent, and the recombinant hCREG glycoprotein is distributed in the lumen of the stent.
[0066] Stent equipped with nanopores: the stent is made of 316L medical stainless steel matrix, and the surface is uniformly equipped with nanopores of about 500nm.
[0067] Preparation of three-sided rapamycin drug stent: Accurately prepare 1% rapamycin drug with acetone solution. The inner surface of the stent is protected, and rapamycin is sprayed on the outer surface of the stent.
[0068] Preparation of CREG protein-eluting scaffold: The recombinant hCREG glycoprotein is fixed to the inner lumen of the scaffold by using the principle of physical adsorption of nanopores. The solvent is a sodium carbonate buffer solution wi...
Embodiment 3
[0069] Example 3 Preparation of double-drug protein combined scaffold
[0070] The surface of the coronary stent in this embodiment is uniformly distributed with recombinant hCREG glycoprotein, rapamycin and paclitaxel. Rapamycin and paclitaxel were distributed on the outer surface of the stent, and recombinant hCREG glycoprotein was distributed in the lumen of the stent.
[0071] Stent equipped with nanopores: the stent is made of 316L medical stainless steel matrix, and the surface is uniformly equipped with nanopores of about 500nm.
[0072] Prepare a three-sided rapamycin drug stent: protect the inner surface of the stent, spray rapamycin and paclitaxel on the outer surface of the stent, the drug concentration is 1%, the drug solvent adopts tetrahydrofuran, and the space between rapamycin and paclitaxel is The ratio is 1:1.
[0073] Preparation of CREG protein-eluting scaffold: The recombinant hCREG glycoprotein is fixed to the inner lumen of the scaffold by using the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com