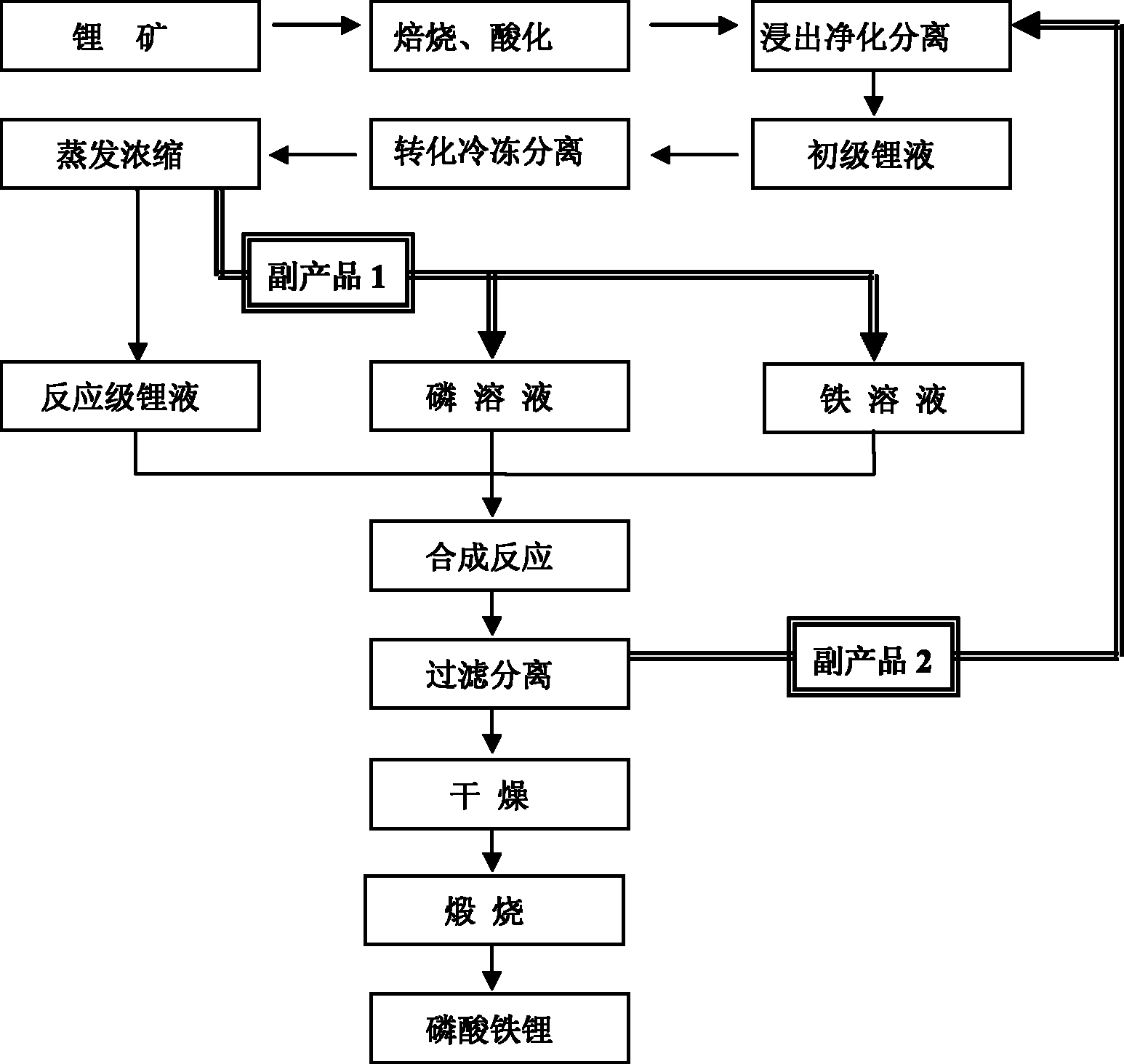
Complete cycle preparation method of lithium iron phosphate by using lithium ores as lithium source
A technology of lithium iron phosphate, cycle preparation, applied in chemical instruments and methods, phosphorus compounds, lithium compounds, etc., can solve the problem of high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Example 1 Preparation of lithium iron phosphate
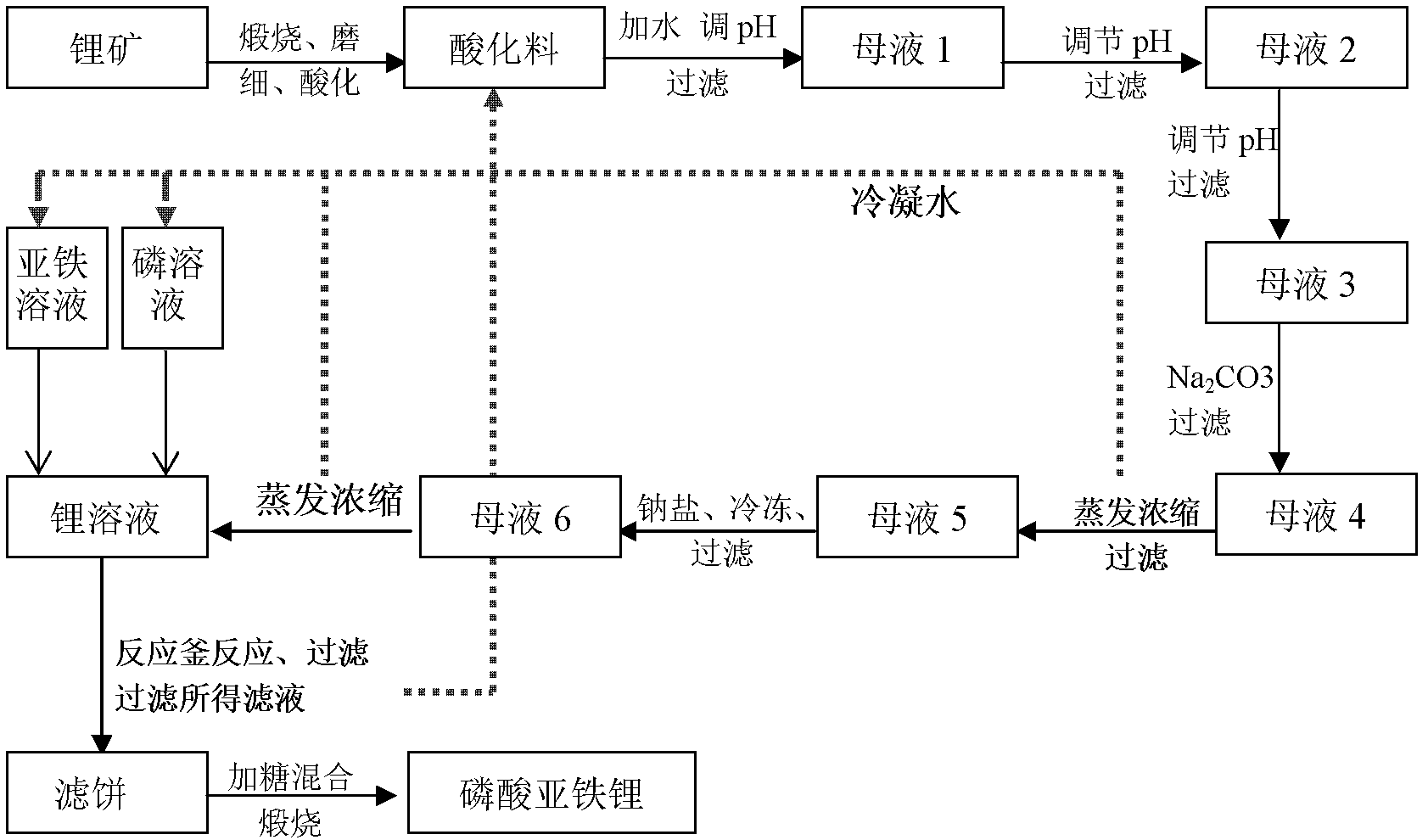
[0132] (1) Weigh 50kg of spodumene, calcinate at 1100°C for 50 minutes, cool, grind finely, add 7.1kg of sulfuric acid (acid to material ratio 1:7) for 50 minutes, and add sulfuric acid to lithium Pour pyroxene powder into 114kg (liquid-solid ratio 2:1) water, adjust the pH value to 5.7 with NaOH, stir for 35 minutes, let stand, and filter to obtain mother liquor 1;
[0133] (2) Adjust the pH value of mother liquor 1 to 8.5 with NaOH, stir and react for 5 minutes, stand still, filter to obtain mother liquor 2; then adjust the pH value of mother liquor 2 to 10.8 with NaOH, stir and react for 5 minutes, stand still, filter to obtain mother liquor 3; Add 236.6 grams of Na in the mother liquor 3 2 CO 3 , stirring and reacting for 30 minutes, standing still, and filtering to obtain mother liquor 4;
[0134] (3) Evaporate and concentrate the mother liquor 4 until its lithium content is 65g / L, leave it still, filter to obt...
Embodiment 2
[0147] Example 2 Preparation of lithium iron phosphate
[0148] (1) Weigh 50 kg of lithium phosphate aluminum, calcined at 1380 ° C for 300 minutes, cooled, ground, added 12.5 kg of sulfuric acid (according to the ratio of acid to material 1:4) for 200 minutes, under stirring conditions, will add acid The spodumene powder was poured into 187.5kg of reclaimed filtrate (liquid-solid ratio 3: 1), and the pH value was adjusted to 6.2 with NaOH, stirred for 50 minutes, left standstill, and filtered to obtain mother liquor 1;
[0149] (2) Adjust the pH value of mother liquor 1 to 9.7 with NaOH, stir and react for 12 minutes, stand still, filter to obtain mother liquor 2; then adjust the pH value of mother liquor 2 to 10 with NaOH, stir and react for 12 minutes, stand still, filter to obtain mother liquor 3; Add 240.5 grams of Na in the mother liquor 3 2 CO 3 , stirred and reacted for 10 minutes, allowed to stand, and filtered to obtain mother liquor 4;
[0150] (3) Evaporate and ...
Embodiment 3-9
[0159] Example 3-9 Preparation of lithium iron phosphate
[0160] The preparation process of Examples 3-9 is the same as that of Example 1, and the required preparation materials and process parameters are shown in Table 2. Simultaneously, the said lithium ore is first made into corresponding lithium salt according to the sulfuric acid method, then adding deionized water to make a lithium solution with a concentration of 25-27g / L, and the rest of the steps for preparing lithium ferrous phosphate are the same as implementing (6)~(10) of example 1, but do not recycle the preparation method of recovering filtrate and condensed water " as comparative example, and comparative example is compared with the production cost of the lithium ferrous phosphate that embodiment 3-9 prepares , the results are shown in Table 2.
[0161] Preparation raw materials and process parameters of table 2 embodiment 3-9
[0162]
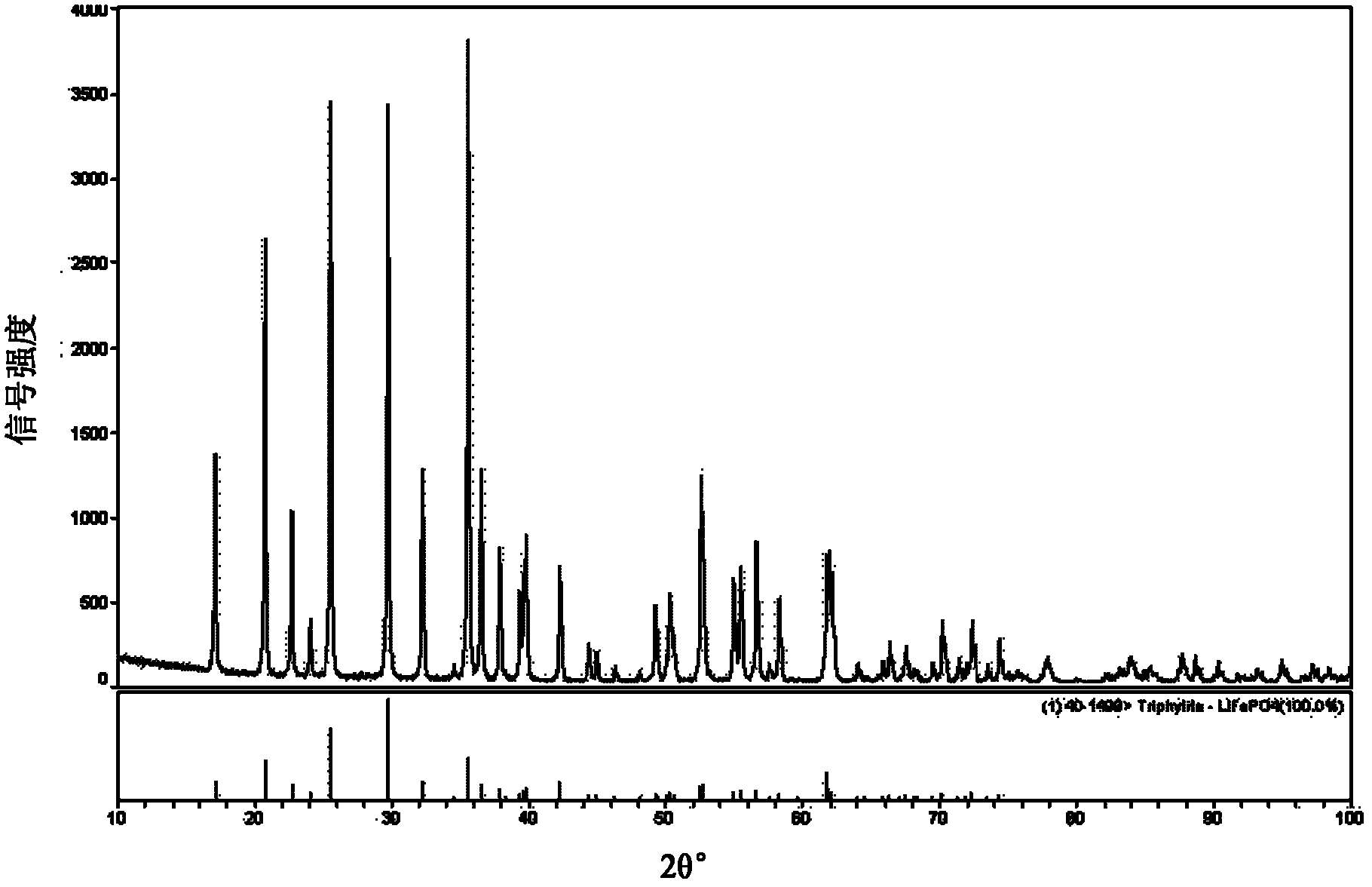
[0163] As can be seen from Table 2, according to the detection met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com