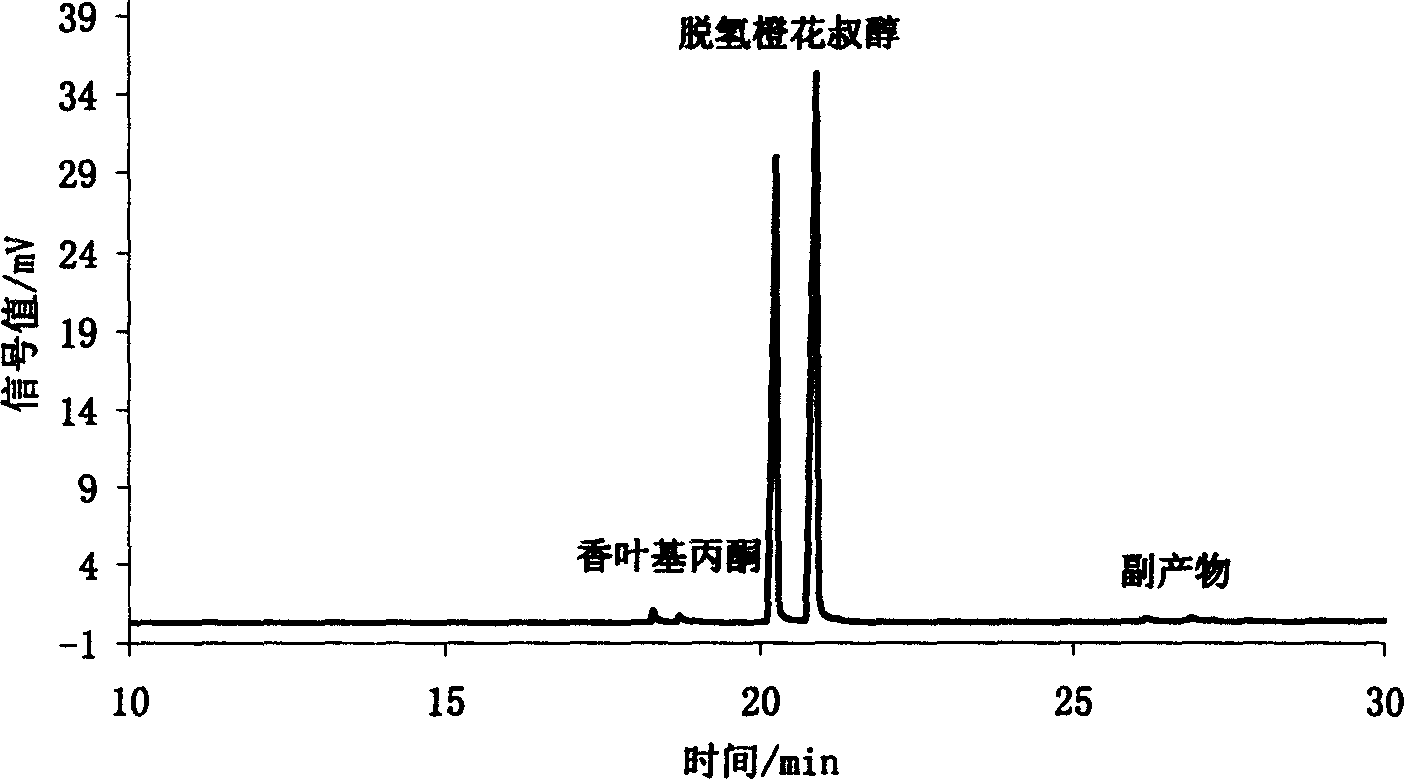
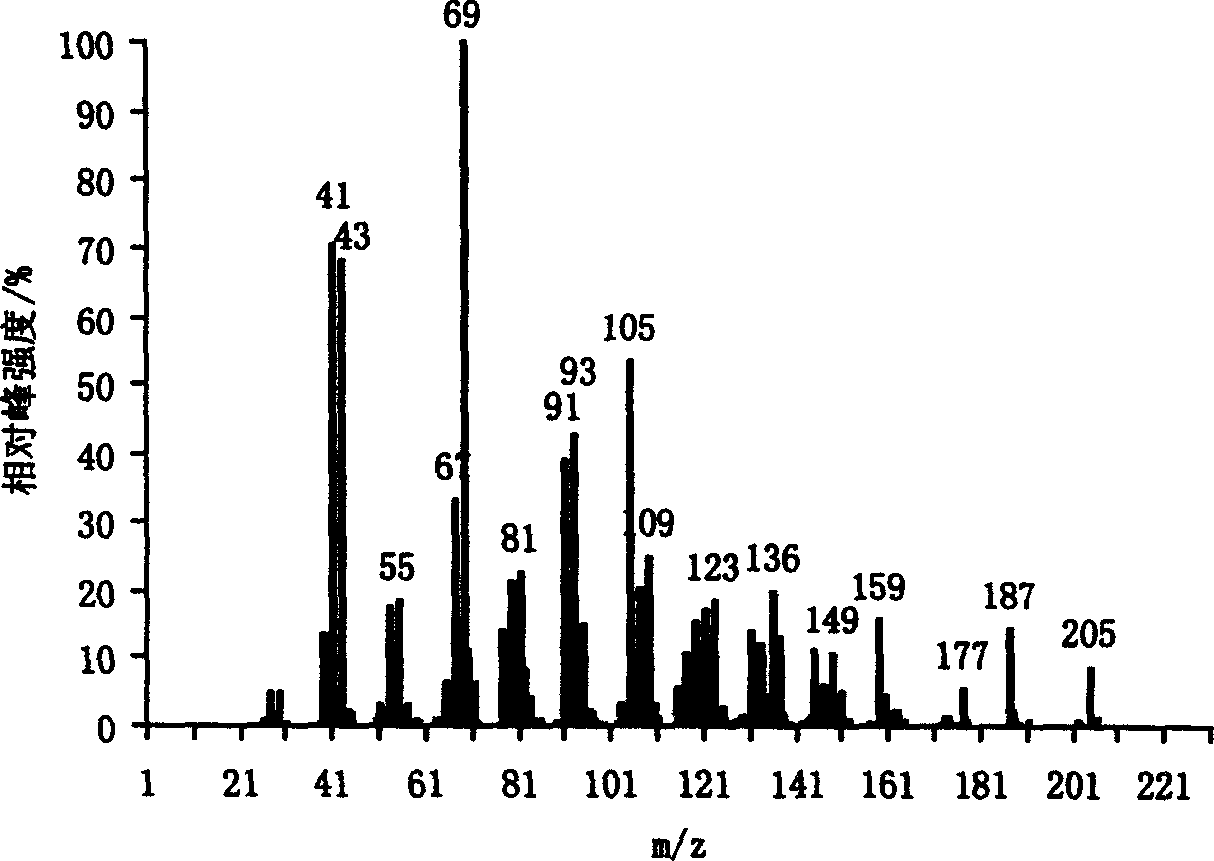
Method for preparing alpha, beta unsaturated alcohol from compound of ketone or aldehyde containing carbonyl
An aldehyde compound and unsaturated technology, which is applied in the preparation of hydroxyl compounds, organic compounds, chemical instruments and methods, etc., can solve the problems of excessive reduction product yield, lower product quality, air pollution, etc., and achieve easy control of the reaction , Improve the purity and quality, and reduce the pollution load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A method for preparing α, β-unsaturated alcohols from carbonyl-containing ketones or aldehydes, the carbonyl-containing ketones or aldehydes can be: general formula R 1 COR 2 Ketone compounds, in this embodiment can be methyl heptenone, geranyl acetone, neryl acetone, farnesyl acetone, phytoketone, cyclohexanone, etc., the general formula is R 1 Aldehyde compounds of CHO, such as citral, geranial, neral, citronellal, vanillin, trimethoxybenzaldehyde, benzaldehyde. In this embodiment, the carbonyl-containing ketone compound is a terpene-based acetone compound, which can obtain better results, and the effect when the terpene-based acetone compound is geranyl acetone, neryl acetone, farnesyl acetone and phytoketone better.
[0047] Ethynylation of carbonyl-containing ketones or aldehydes:
[0048] The first step: put a strong base compound equivalent to 1 to 5 times the molar mass of carbonyl-containing ketones or aldehydes and an organic solvent equivalent to 5 to 15 t...
Embodiment 2
[0059] Preparation of dehydronerolidol with geranylacetone as raw material:
[0060] Add 0.35mol potassium hydroxide powder and 200mL organic solvent (tetrahydrofuran or toluene) to a 250mL four-necked bottle, place in a liquid bath at -20 to 10°C and stir to make the temperature reach internal and external equilibrium, and then pass through acetylene gas to replace and remove the reaction. The air in the container is then continuously fed with acetylene gas. The reaction is carried out in a simple airtight balloon system with a pressure of 0.1-0.25 MPa to avoid the overflow and loss of acetylene gas. After 0.5 hours, slowly drop through the constant pressure dropping funnel. Add 0.1mol (19.4g, about 22.3mL, if necessary, dilute with a little solvent) geranyl acetone. After the dropwise addition, continue to keep warm and stir until the reaction is over. The reaction time is 0.5~100h. During the reaction, keep the reactants in the reactor in an acetylene atmosphere with a pres...
Embodiment 3
[0064] Other conditions in Implementation 2 are unchanged, only the solvent used is changed, and then the same ethynylation reaction is carried out. The obtained results are shown in Table 1.
[0065] no
[0066] All hydrogenation reaction conditions in embodiment 2 do not change, carry out the same selective hydrogenation reaction as embodiment 2, make nerolidol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com