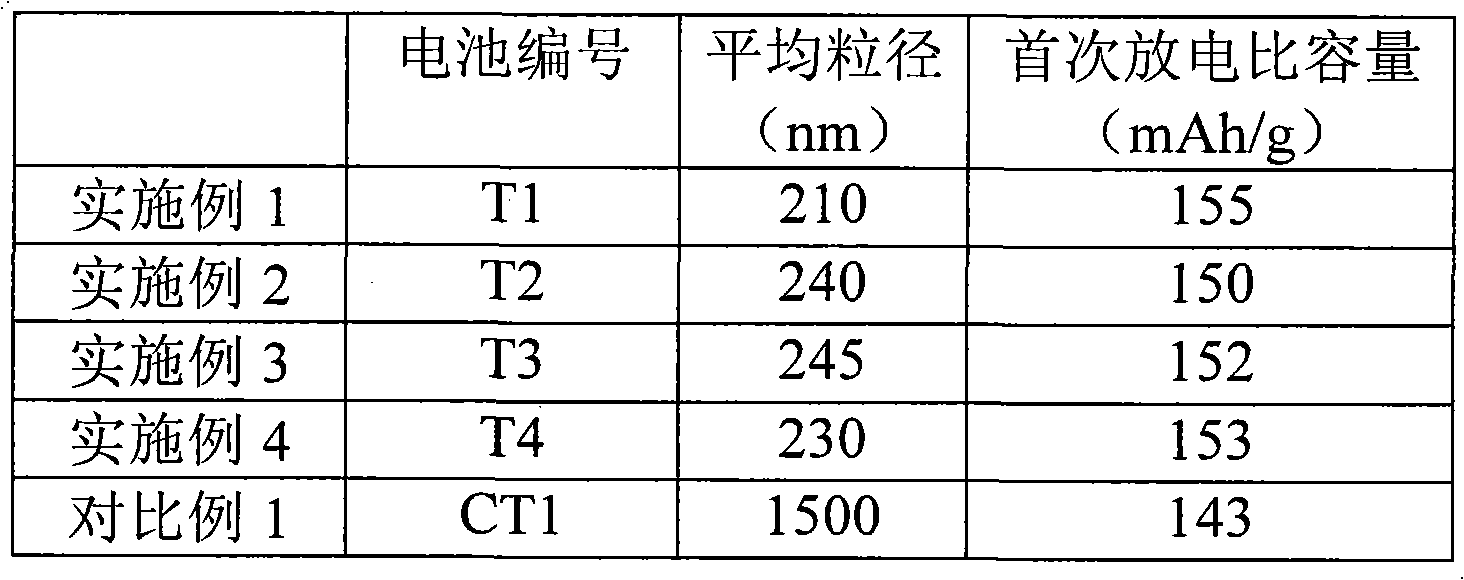
Cathode active substance for lithium ion secondary battery, preparation method and lithium ion secondary battery
A negative electrode active material and secondary battery technology, which is applied to secondary batteries, battery electrodes, circuits, etc., can solve the problems of complex preparation methods, small specific capacity of the first discharge of batteries, and large average particle size of lithium chromium titanate. The effect of simple production process and high initial discharge specific capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] The invention provides a method for preparing a negative electrode active material containing nanometer lithium chromium titanate, the method comprising:
[0017] 1) After mixing titanium dioxide, chromium trioxide, a lithium source and a solvent, drying and removing the solvent to obtain a first mixture;
[0018] 2) mixing and drying the first mixture obtained in step 1 with a saturated solution of low-temperature molten salt to obtain a second mixture;
[0019] 3) Calcining the second mixture obtained in step 2 to remove the low-temperature molten salt, wherein the low-temperature molten salt is a salt that can melt under calcination conditions and does not react with other components in the mixture.
[0020] In step 1, the molar ratio of titanium dioxide, dichromium trioxide and lithium source is 2:1-2:1.05, and in a preferred case, the molar ratio of titanium dioxide, dichromium trioxide and lithium source is 2:1:1.05.
[0021] The method of mixing titanium dioxide...
Embodiment 1
[0035] (1) Preparation of nano-chromium lithium titanate
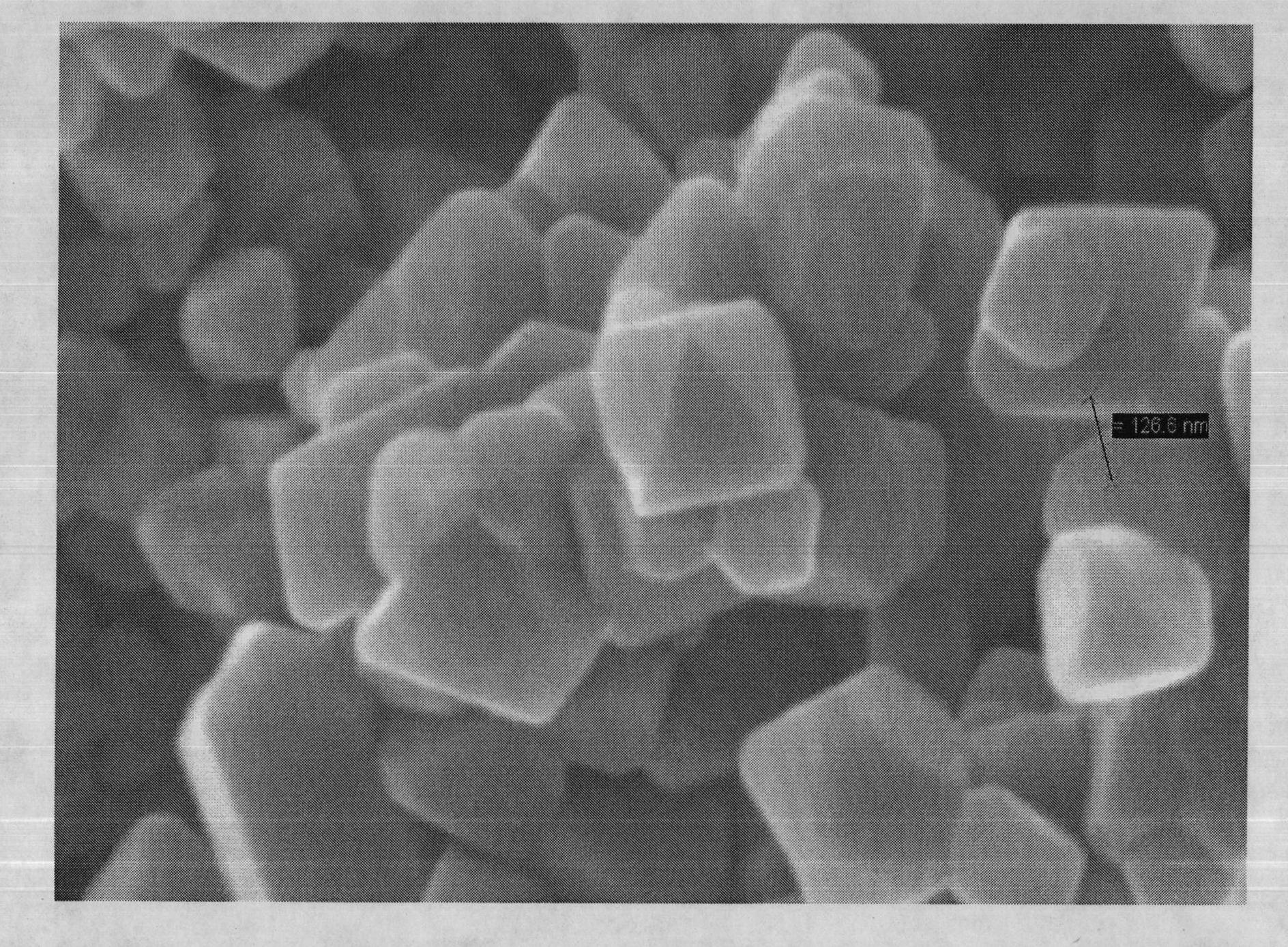
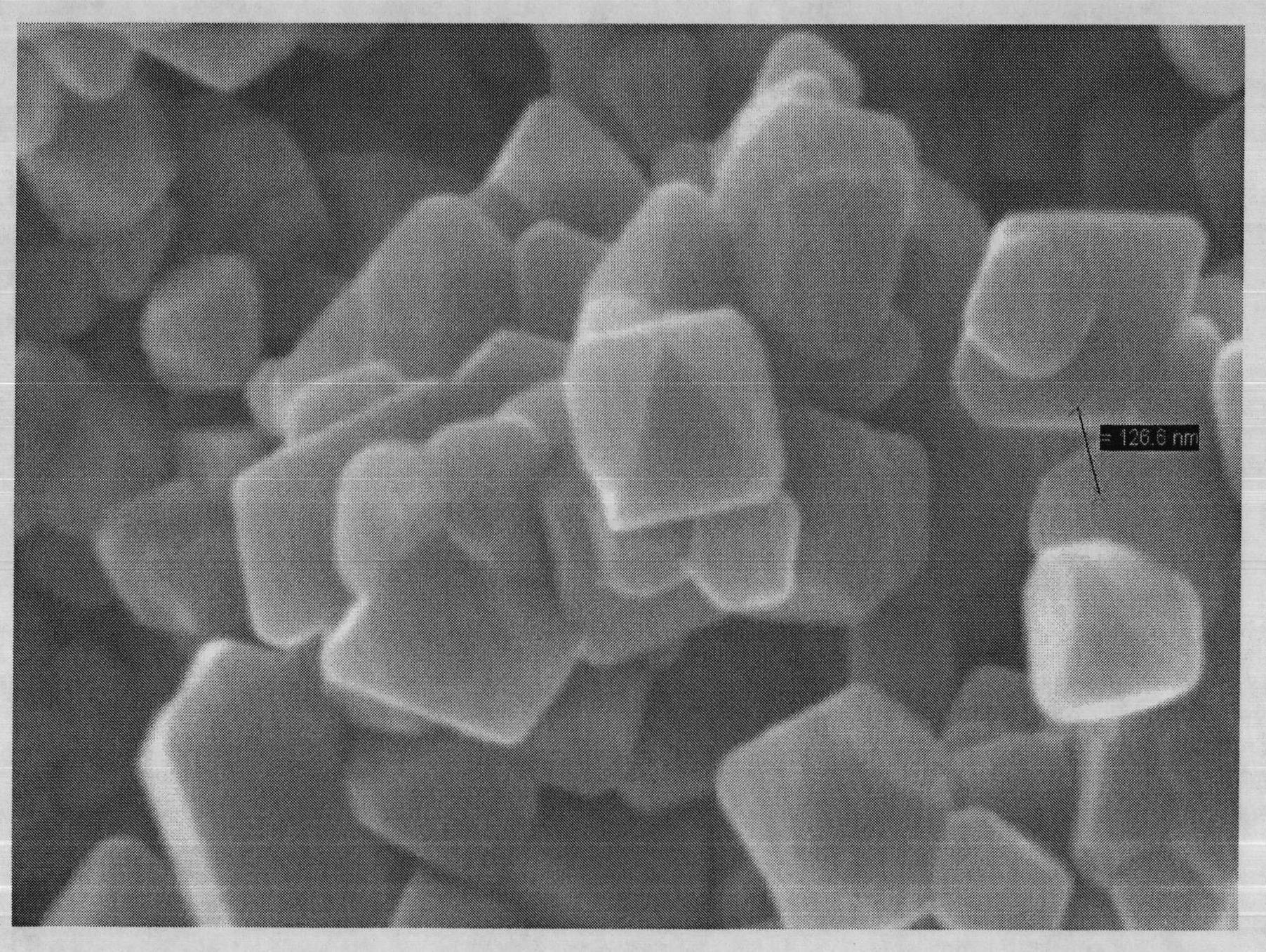
[0036] TiO 2 、Cr 2 o 3 and Li 2 CO 3According to the ratio of 2:1:1.05, put it in a 100ml ball mill jar, add 80ml of absolute ethanol, ball mill for 8 hours, and mix thoroughly. Dry and remove dehydrated alcohol, obtain the first mixture, the first mixture is dissolved in the saturated solution that is solute with LiCl again, wherein the weight ratio of the first mixture and LiCl is 1: 3, in a stirrer (ELE Yi Le electromechanical, EBF -22) Stir for 5 minutes at a rotational speed of 1100r / min, mix well, and dry at 100°C for 24 hours to obtain a second mixture, heat the second mixture to 600°C to melt the salt, and After 12 h at temperature, the Li was removed by repeated washing with distilled water. + and Cl - , and the washed sample was dried in a vacuum oven at 100° C. for 24 hours to obtain nano-chromium lithium titanate sample A1. The morphology of the synthesized product was observed with a JSM-5610LV sca...
Embodiment 2
[0045] (1) Preparation of nano-chromium lithium titanate
[0046] TiO 2 、Cr 2 o 3 and Li 2 CO 3 Put it in a ball mill jar according to the ratio of 2:1:1, add it into a 100ml ball mill jar at the same time, add 80ml of absolute ethanol as a solvent, ball mill for 8 hours, mix thoroughly, dry and remove the solvent to obtain the first mixture. Dissolving the first mixture in a saturated solution with KCl as the solute, wherein the weight ratio of the first mixture to KCl is 1:4, and then drying at 100° C. for 24 hours to obtain a second mixture, which is heated to Melt KCl at 700°C, and then keep it at 700°C for 12 hours, then wash repeatedly with distilled water to remove K + and Cl - , and the washed sample was dried in a vacuum oven at 100° C. for 24 hours to obtain nano-chromium lithium titanate sample A2. Observing the morphology of the synthesized product with a JSM-5610LV scanning electron microscope (SEM), the product is an octahedral structure with an average pa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com