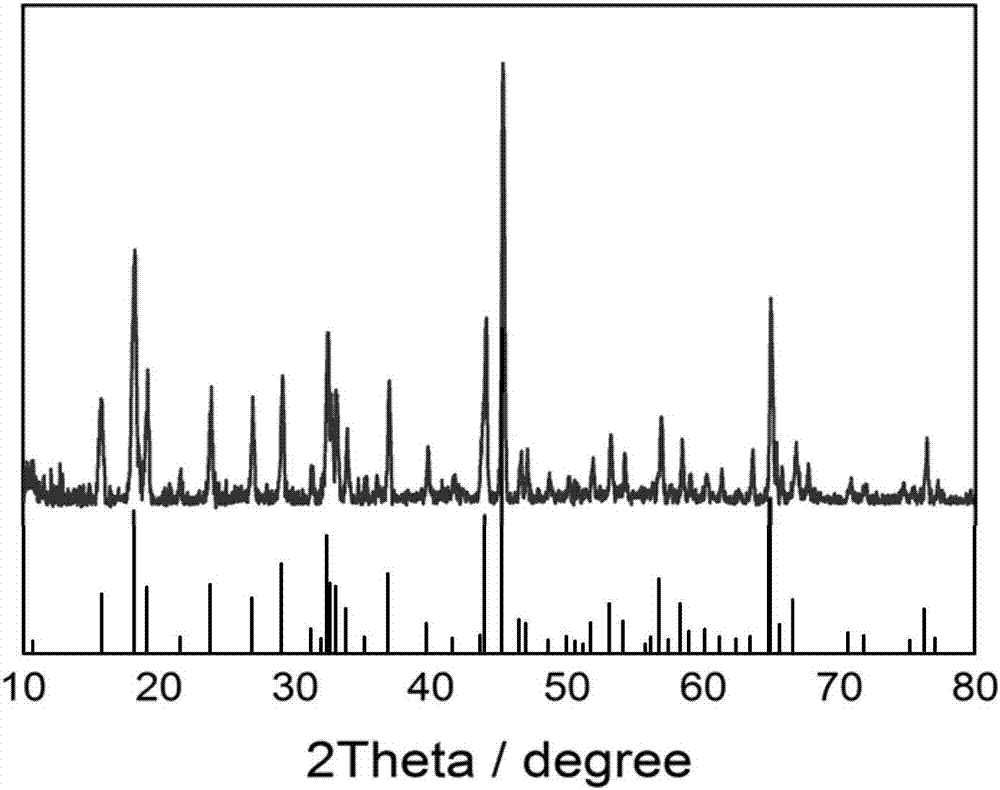
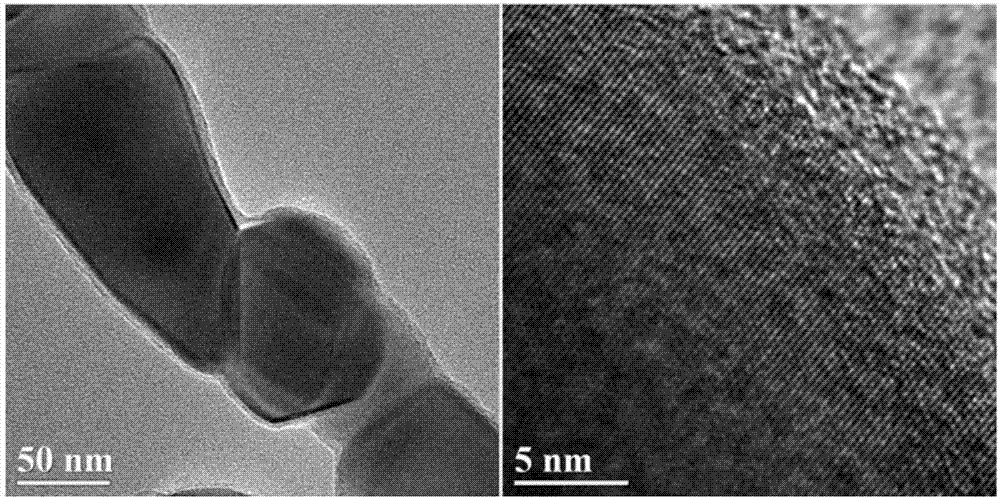
Carbon-coated Na2Li2Ti6O14 nanofiber and preparation method thereof
A nanofiber and carbon coating technology, applied in the direction of nanotechnology, nanotechnology, fiber chemical characteristics, etc., can solve the problems of poor electrical conductivity, restricted application, electrical conductivity needs to be improved, etc., to achieve excellent performance, high initial discharge specific capacity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 0.5mmol of Na 2 CO 3 and 3.0mmol of isopropyl titanate were dissolved in 20mL of N,N-dimethylformamide (DMF) to form solution A; 1.0mmol of LiCH 3 Dissolve COO in a mixed solvent of 10mL absolute ethanol and 3mL glacial acetic acid to form solution B; mix solutions A and B evenly, add 0.6g polyvinylpyrrolidone (PVP), stir for 10h, and form a clear mixed solution. The molar ratio of Na, Li and Ti is 1:1:3, and the concentration of polyvinylpyrrolidone (PVP) is 0.018g / mL; % atmosphere for electrospinning; put the obtained electrospun product in a 60°C oven for 24 hours; transfer the dried electrospun product to a muffle furnace and sinter at 800°C for 3 hours in an air atmosphere to obtain Na 2 Li 2 Ti 6 o 14 nanofibers; the sintered Na 2 Li 2 Ti 6 o 14 Nanofibers and sucrose were placed in two corundum boats respectively, and then sequentially placed in a tube furnace, at N 2 Sintered at 500°C for 8 hours in the atmosphere, N 2 It enters from the end of the co...
Embodiment 2
[0032] 3.0mmol of NaCH 3 COO and 9.0 mmol of tetrabutyl titanate were dissolved in 40 mL of N,N-dimethylformamide (DMF) to form solution A; 1.5 mmol of Li 2 CO 3 Dissolve in a mixed solvent of 10mL absolute ethanol and 4mL glacial acetic acid to form solution B; mix solutions A and B evenly, add 0.8g polyvinylpyrrolidone (PVP), and stir for 24h to form a clear mixed solution. Na in the solution , the molar ratio of Li to Ti element is 3:3:9, the concentration of polyvinylpyrrolidone (PVP) is 0.015g / mL; The clear solution is 70% under voltage 20kV and flow rate 1.0mL / h, relative humidity Electrospinning was carried out under an atmosphere; the obtained electrospun products were dried in an oven at 90 °C for 6 h; the dried electrospun products were transferred to a muffle furnace and sintered at 600 °C for 8 h in an air atmosphere to obtain Na 2 Li 2 Ti 6 o 14 nanofibers; the sintered Na 2 Li 2 Ti 6 o 14 Nanofibers and glucose were placed in two corundum porcelain boats...
Embodiment 3
[0034] 2.0mmol of NaCH 3 COO and 6.0 mmol of tetrabutyl titanate were dissolved in 30 mL of N,N-dimethylformamide (DMF) to form solution A; 1.0 mmol of Li 2 CO 3 Dissolve in a mixed solvent of 10mL absolute ethanol and 4mL glacial acetic acid to form solution B; mix solutions A and B evenly, add 0.7g polyvinylpyrrolidone (PVP), stir for 12h, and form a clear mixed solution. Na in the solution , the molar ratio of Li to Ti element is 2:2:6, the concentration of polyvinylpyrrolidone (PVP) is 0.016g / mL; The clarified solution is 60% under voltage 15kV and flow rate 0.5mL / h, relative humidity Electrospinning was carried out under an atmosphere; the obtained electrospun product was dried in an oven at 80 °C for 12 h; the dried electrospun product was transferred to a muffle furnace and sintered at 700 °C for 5 h in an air atmosphere to obtain Na 2 Li 2 Ti 6 o 14 nanofibers; the sintered Na 2 Li 2 Ti 6 o 14 Nanofibers and cellulose were placed in two corundum porcelain boat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com