Preparation method of hydroxy fatty acid glyceride
A technology of hydroxy fatty acid glyceride and glycerin, which is applied in the field of ester preparation, to achieve the effects of reducing reaction tendency, increasing transesterification rate, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] In one embodiment, the preparation method of hydroxy fatty acid glyceride comprises the following steps:
[0038] Mixing step: add lactone, glycerin with 0.5-10 times the amount of lactone substance, water with 0.05-10 times the amount of lactone substance, main solvent with 0-200 times the amount of lactone substance, 0 An auxiliary solvent of ~20 times the amount of the lactone substance, and a lipase of 0.01%-5.0% by weight of the lactone;
[0039] Reaction steps: react at 30 to 80° C., and obtain a reaction liquid containing hydroxy fatty acid glyceride after reaching an equilibrium state.
[0040] Wherein, the main solvent refers to an organic solvent capable of dissolving at least one of lactone and glycerol, generally a hydrophilic solvent, which can be one or more of tert-butanol, tert-amyl alcohol, acetone and ionic liquid Multiple mixing, described auxiliary solvent refers to the organic solvent that can promote the dissolubility of reaction substrate in main...
Embodiment 1
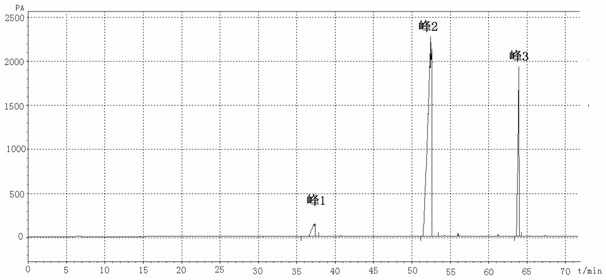
[0054] Add 170.250 g (1 mol) δ-decalactone, 920.090 g (10 mol) glycerol, 18.016 g (1 mol) water, 7412.160 g (100 mol) tert-butanol and 7.812 g (0.1 mol) dimethyl sulfoxide to the enzyme reactor Add 5g of immobilized Candida lipase, and stir the reaction at 60°C for 24 hours. The reaction basically reaches equilibrium, and 5-hydroxycapric acid glyceride (monoester and diester) is produced, and the remaining unreacted δ - Decalactone 112.458g (0.66mol), the transesterification rate of δ-decalactone reached 34%.
[0055] Finish the reaction, inactivate the enzyme, remove the lipase by filtration, carry out vacuum distillation on the reaction liquid to remove tert-butanol, water, most of dimethyl sulfoxide and glycerin, and put the distillation residue under the conditions of temperature 50°C and vacuum degree 200Pa Carry out molecular distillation, glycerol, dimethyl sulfoxide and part of δ-decalactone are distilled out, and the remaining part of molecular distillation is extract...
Embodiment 2
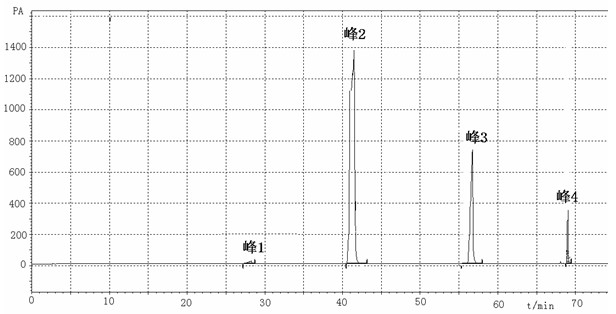
[0057] Add 114.140g (1mol) γ-caprolactone, 92.090g (1mol) glycerin, 1.802g (0.1mol) water and 580.800g (10mol) acetone to the enzyme reactor, then add 0.2g porcine pancreatic lipase, The reaction was stirred at 50°C for 96 hours, and the reaction basically reached equilibrium, producing 4-hydroxycaproic acid glyceride (monoester, diester, triester), and the remaining unreacted γ-caprolactone 51.071g (0.45mol), γ -The transesterification rate of caprolactone reaches 55%.
[0058] Finish the reaction, inactivate the enzyme, remove the lipase by filtration, carry out normal pressure and reduced pressure distillation on the reaction solution to remove acetone, water, part of γ-caprolactone and glycerin, and put the distillation residue under the conditions of temperature 80°C and vacuum degree 20Pa Molecular distillation is carried out, glycerin, γ-caprolactone and 4-hydroxycaproic acid glyceride are distilled, and the distilled part is subjected to silica gel column chromatograph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com