Preparation method of 2-arachidonic acid monoglyceride
A technology of tetraenoic acid glycerol and arachidonic acid, applied in the direction of fermentation and the like, can solve the problems of lack of specificity, unsatisfactory high purity, restrictions, etc., and achieve high purity, reduced production cost, and high transesterification rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
[0030] Embodiment 1~5 is the embodiment that is rich in arachidonic acid triglyceride prepared for transesterification:
Embodiment 1
[0031] Embodiment 1: (select the different kinds of enzymes to compare)
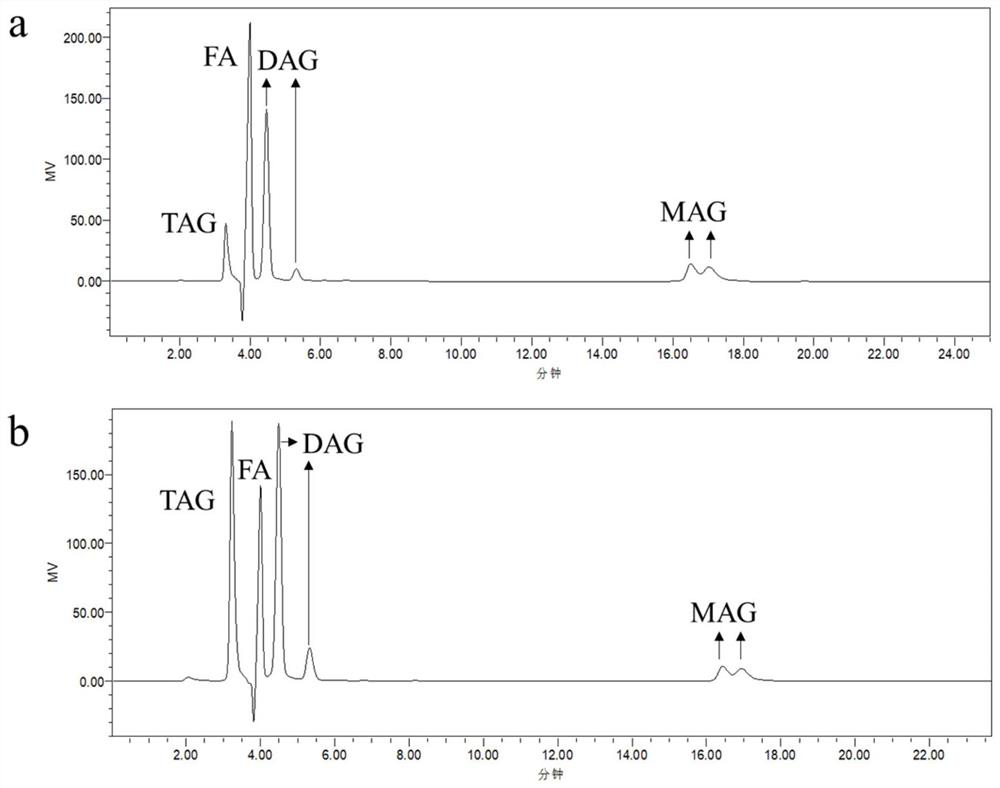
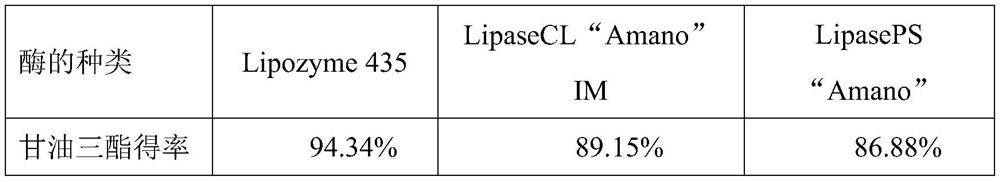
[0032] Add 15mmol ethyl arachidonic acid and 4mmol glycerin to a 25mL Erlenmeyer flask, then add lipase, the amount of enzyme added (relative to the mass fraction of fatty acid ethyl ester) is 10%, and the system is mixed evenly, and the Enzyme-catalyzed reaction under pressure, magnetic stirring at 220r / min for 6h; after the reaction, centrifuge at 4000r / min to get the supernatant, remove the lipase, and get the lipid composition in the reaction crude product by HPLC-RID detection, calculate the glycerol The yield of triester, the result is shown in Table 1.
[0033] Table 1. The effect of different enzyme types on the yield of triglyceride in the enzymatic reaction
[0034]
Embodiment 2
[0035] Embodiment 2: (different molar ratios of substrates are compared)
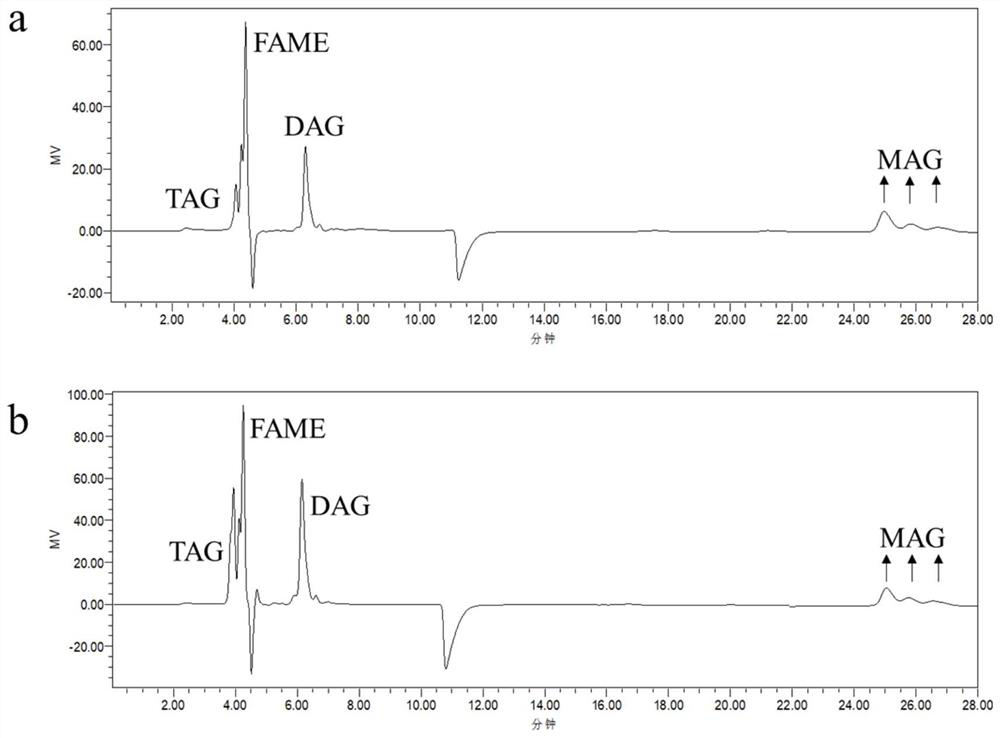
[0036] Add 15mmol ethyl arachidonic acid and a certain amount of glycerin to a 25mL Erlenmeyer flask, the ratio of ethyl arachidonic acid and glycerol is 3:0.5~3:1, then add Lipozyme 435 lipase (from Candida antarctica), the enzyme The addition amount (relative to the mass fraction of fatty acid ethyl ester) was 10%. After the system was mixed uniformly, the enzymatic reaction was carried out at 55°C and 200Pa pressure, and the reaction was carried out by magnetic stirring at 220r / min for 6h. After the reaction, the supernatant was centrifuged at a speed of 4000r / min to remove lipase, and the lipid composition in the reaction crude product was obtained by HPLC-RID detection, and the yield of triglyceride was calculated. The results are shown in Table 2.
[0037] Table 2. Effects of different substrate molar ratios on triglyceride yield in enzymatic reactions
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com