Nickel-based catalyst as well as preparation method and application thereof
A nickel-based catalyst and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve disasters, gas blockage and other problems, and achieve anti-carbon deposition Strong ability, low cost, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
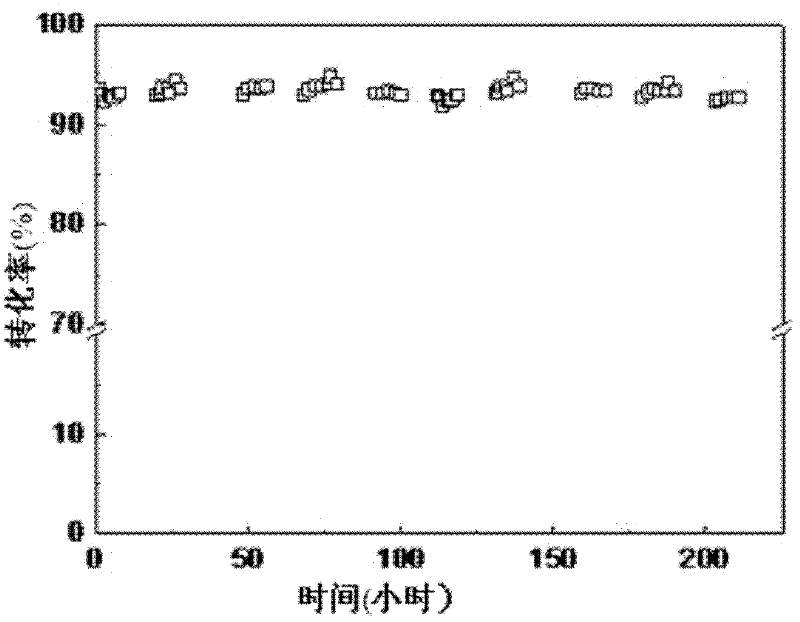
Examples
Embodiment 1
[0029] Weigh 1.17g of cerium nitrate and dissolve it in water to form an aqueous solution of cerium nitrate; dry 10.00g of γ-Al at room temperature 2 o 3 Immerse in the prepared cerium nitrate aqueous solution for 12 hours by equal volume impregnation method; dry at 120°C for 24 hours; bake at 450°C for 2 hours in air atmosphere to obtain the modified carrier.
[0030] Weigh 5.74g of nickel nitrate and dissolve it in water to form an aqueous solution of nickel nitrate; at room temperature, the prepared modified carrier is impregnated in the prepared aqueous solution of nickel nitrate for 12 hours by an equal volume impregnation method; dried at 120°C for 24 hours; Calcined at 500° C. for 5 hours in an atmosphere to obtain a catalyst precursor.
[0031] The prepared catalyst precursor was reduced in hydrogen at 900 °C for 1 hour.
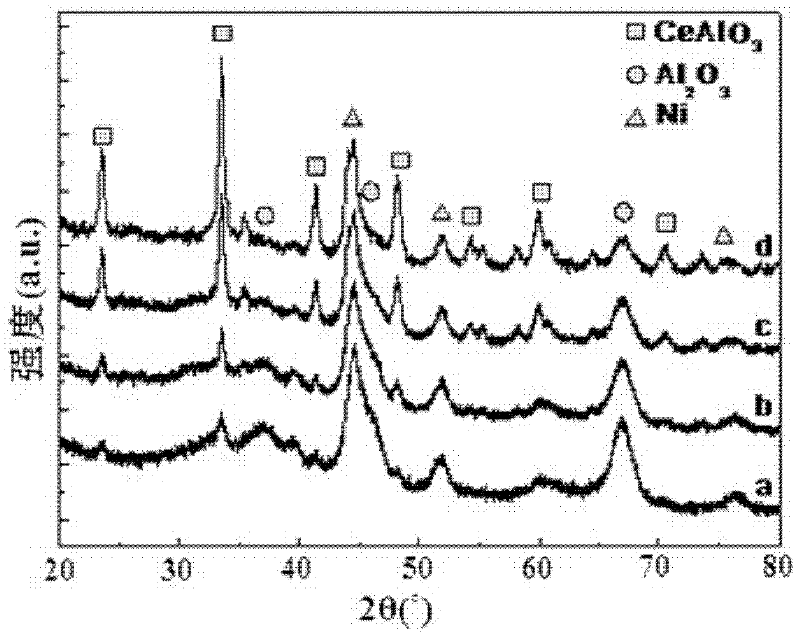
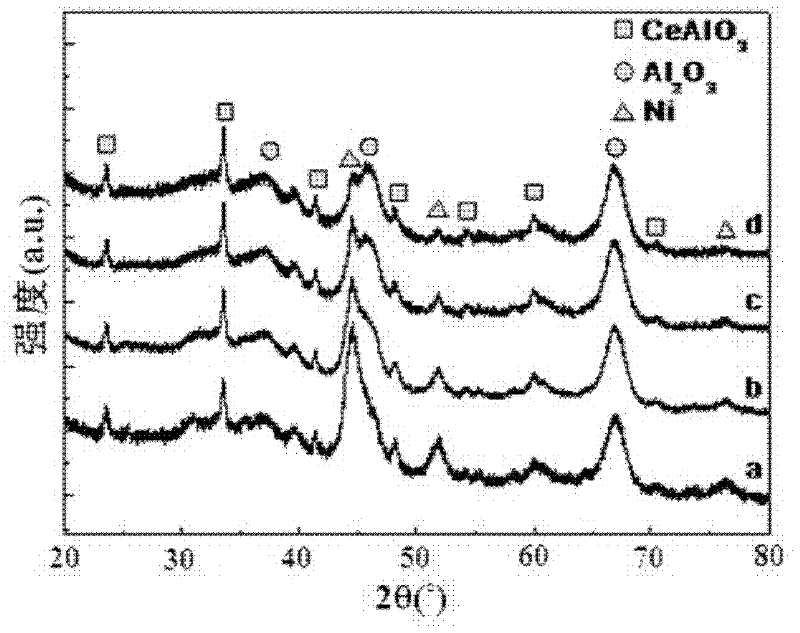
[0032] figure 1 (a) is the XRD collection of collections of catalysts that the present embodiment makes, by figure 1 (a) It can be seen that the...
Embodiment 2
[0039] Weigh 2.45g of cerium nitrate and dissolve in water to form an aqueous solution of cerium nitrate; dry 10.00g of γ-Al at room temperature 2 o 3 Immerse in the prepared cerium nitrate aqueous solution for 12 hours by equal volume impregnation method; dry at 120°C for 24 hours; bake at 450°C for 2 hours in air atmosphere to obtain the modified carrier.
[0040] Weigh 6.02g of nickel nitrate and dissolve it in water to form an aqueous solution of nickel nitrate; at room temperature, impregnate the prepared modified carrier in the prepared aqueous solution of nickel nitrate by an equal volume impregnation method for 12 hours; dry at 120°C for 24 hours; Calcined at 500° C. for 5 hours in an atmosphere to obtain a catalyst precursor.
[0041] The prepared catalyst precursor was reduced in hydrogen at 900 °C for 1 hour.
[0042] figure 1 (b) is the XRD collection of illustrative plates of the prepared catalyst of the present embodiment, by figure 1 (b) It can be seen that ...
Embodiment 3
[0049] Weigh 3.85g of cerium nitrate and dissolve in water to form an aqueous solution of cerium nitrate; dry 10.00g of γ-Al at room temperature 2 o 3 Immerse in the prepared cerium nitrate aqueous solution for 12 hours by equal volume impregnation method; dry at 120°C for 24 hours; bake at 450°C for 2 hours in air atmosphere to obtain the modified carrier.
[0050] Weigh 6.31g of nickel nitrate and dissolve in water to form nickel nitrate aqueous solution; at room temperature, the prepared modified carrier is impregnated in the prepared nickel nitrate aqueous solution for 12 hours by equal volume impregnation; dry at 120°C for 24 hours; Calcined at 500° C. for 5 hours in an atmosphere to obtain a catalyst precursor.
[0051] The prepared catalyst precursor was reduced in hydrogen at 900 °C for 1 hour.
[0052] figure 1 (c) is the XRD collection of collections of catalysts that the present embodiment makes, by figure 1 (c) It can be seen that the prepared catalyst is compo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com