Method for preparing dimethyl ether through methanol dehydration
A technology of methanol dehydration and dimethyl ether, which is applied in the directions of dehydration of hydroxyl-containing compounds to prepare ether, ether preparation, chemical instruments and methods, etc., can solve the problems of easy deactivation, easy carbon deposition, and many by-products, etc. The effect of improving, strong anti-carbon deposition ability and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Catalyst preparation:
[0049] Catalyst A preparation
[0050] Preparation of the carrier:
[0051] Weigh 316g of cetyltrimethylammonium bromide and 62.4g of citric acid to form a mixed solution, add 246mL tetraethyl orthosilicate into the mixed solution, stir for 2 h, and then stir at 70°C until coagulation The gel was aged at room temperature for 12 h, then dried at 110 °C for 8 h, and calcined at 600 °C for 3 h to obtain a silica support, in which the molar ratio of cetyltrimethylammonium bromide to silica was 0.8, and the molar ratio of citric acid to silicon oxide is 0.3. The nature of the carrier is: the specific surface area is 612 m 2 / g, the pore volume is 0.76 mL / g, and the average pore diameter is 5.0 nm.
[0052] Catalyst preparation:
[0053] Add the prepared silica carrier into a C6 alkane solvent, soak it for 10min, then filter, and dry at room temperature until there is no liquid phase on the surface of the carrier; then add it into an aqueous s...
Embodiment 2
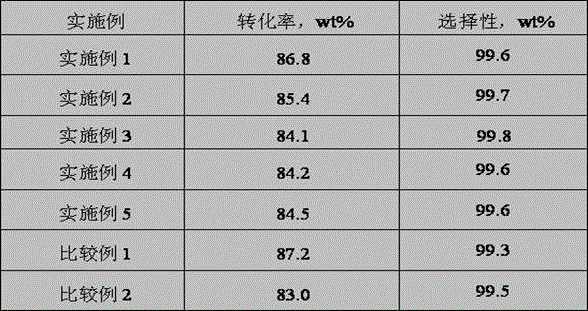
[0059] Catalyst A and B loading volume ratio are selected as 1:1 in embodiment 1, and reaction condition is pressure 1.5MPa, mass space velocity 2.4h -1 , the reaction temperature was 180 °C, and other evaluation conditions remained unchanged. The results of conversion and DME selectivity are shown in Table 1.
Embodiment 3
[0061] Catalyst A and B packing volume ratio are 1:3 among the selection embodiment 1, and reaction condition is pressure 3.0MPa, mass space velocity 3.0h -1 , the reaction temperature was 190 °C, and other evaluation conditions remained unchanged. The results of conversion and dimethyl ether selectivity are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com