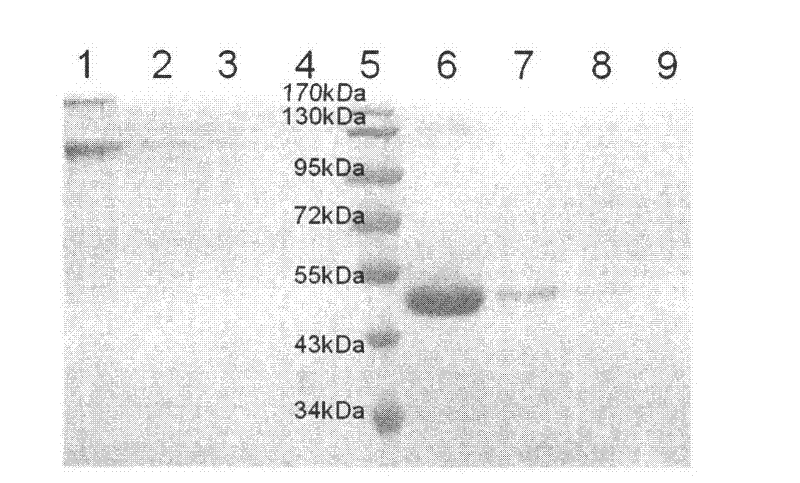
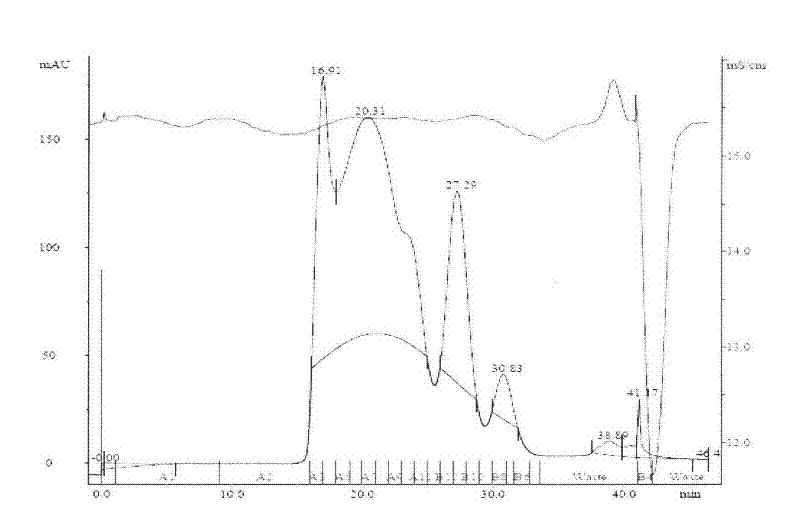
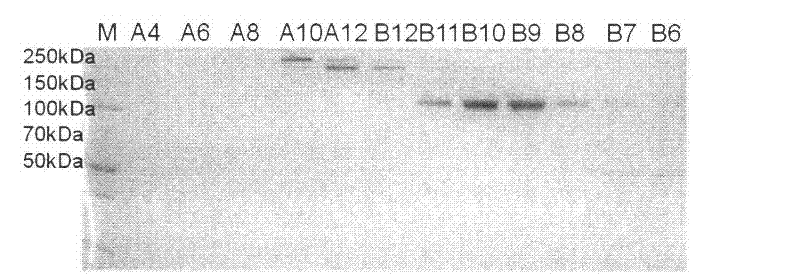
Experimental technology for Fc segment fusion gene (VEGF165-Fc) of recombinant human vascular endothelial growth factor 165 and immune globulin
A technology of v165-fc and protein, which is applied in the experimental technical field of recombinant human vascular endothelial growth factor 165 and immunoglobulin Fc fragment fusion protein (VEGF165-Fc), can solve the problem that the activity and toxin content are difficult to meet clinical diagnosis and treatment, Problems such as the low degree of glycosylation of protein products, to achieve high expression levels, improve product yield and quality, and reduce costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] 1. Preparation of Plasmids for Transfection
[0025] The expression plasmid pR-VEGF165-Fc linked with the VEGF165-Fc gene required for transfection was prepared with an endotoxin-removing plasmid purification kit (purchased from Qiagen). See the instruction manual for the method. Dissolve the plasmid with 200 μl TE buffer, and measure its concentration with a UV spectrophotometer.
[0026] 2. Preparation of PEI for transfection
[0027] 1) Pour 450ml of ultrapure water prepared by Milli-Q into a 500ml beaker;
[0028]2) Weigh 500 mg linear PEI (purchased from Poly Science Company) on the balance, add it to the beaker, and stir it continuously with a magnetic stirrer;
[0029] 3) Add pre-prepared 12M concentrated HCI dropwise to make the pH<2.0 (about 800 μl is needed). Stir the PEI solution for about 2-3 hours;
[0030] 4) Add pre-prepared 10M NaOH solution to adjust the pH to 7.0 (about 500 μl is required). Dilute the volume of PEI solution to 500ml with Milli-Q ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com