Nano-micro drug delivery system and preparation method of 10-hydroxyl camptothecin
A hydroxycamptothecin system technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of repeated droplet breakage, low drug loading, and uncontrollable , to achieve the effect of increasing the embedding rate, improving the drug effect, and stabilizing the dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
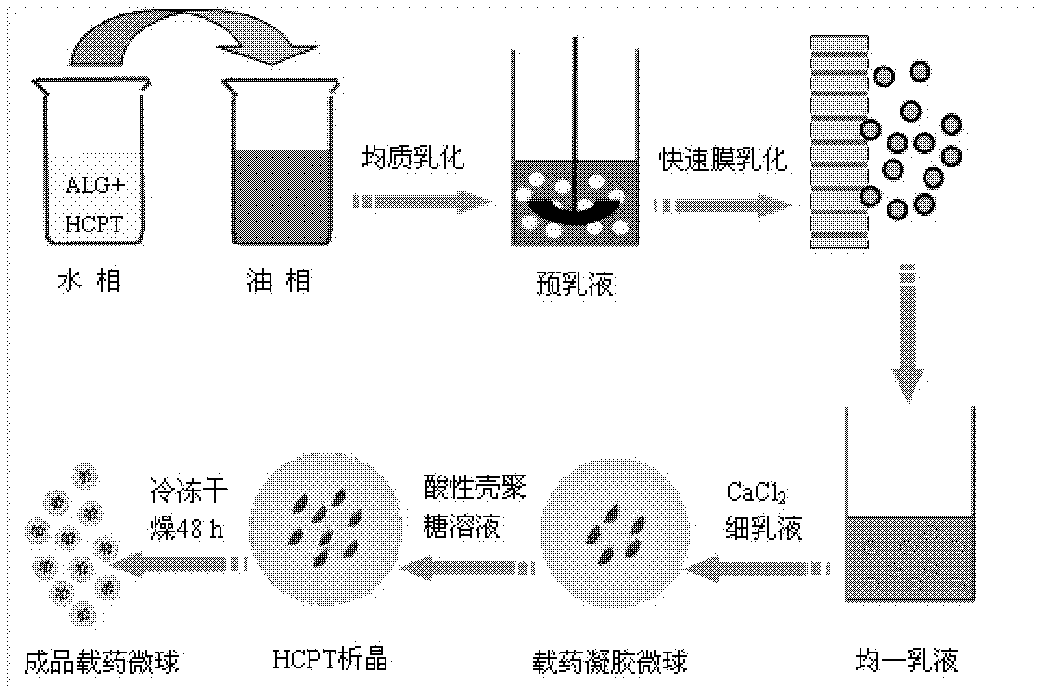
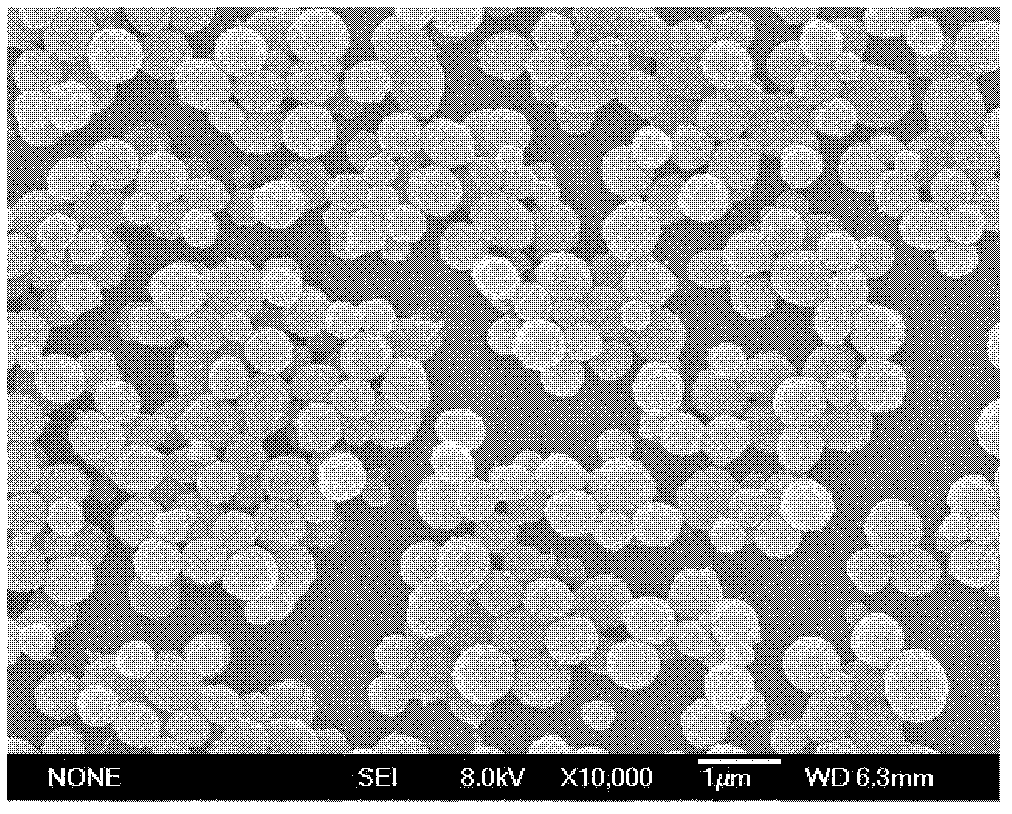
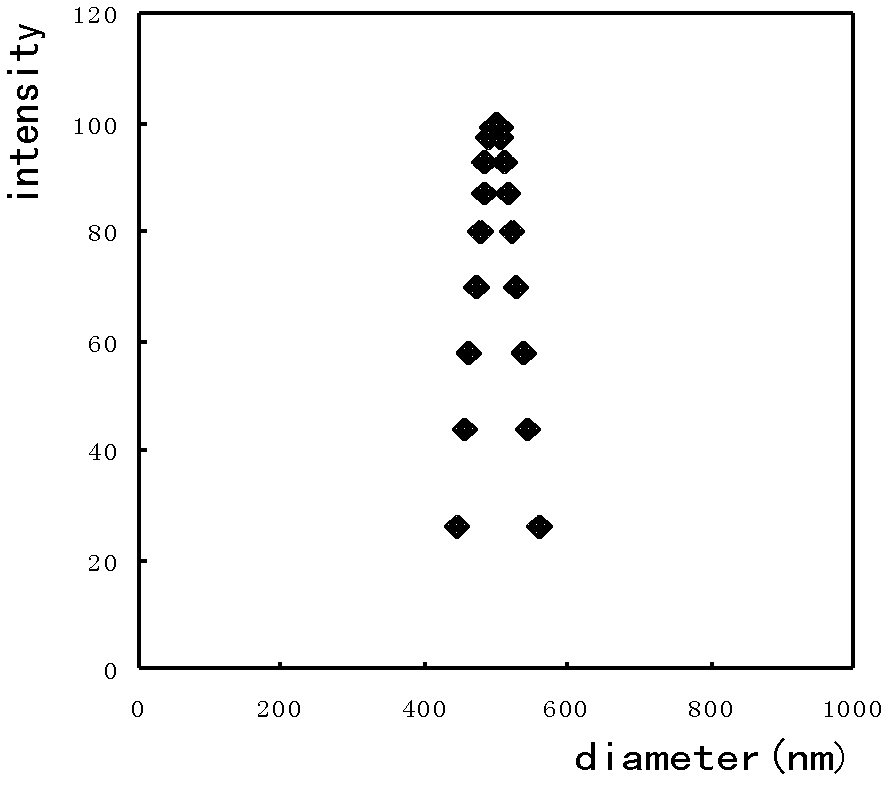
[0059] In the experiment, a microporous membrane with a pore size of 2.8 μm was used to prepare drug-loaded gel microspheres. The specific implementation was as follows: the hydrophobic membrane with a pore size of 2.8 μm was soaked in a lipophilic substance, so that the porous membrane was fully wetted to ensure the drug-loaded gel microspheres on the membrane. The hydrophobic chain is completely stretched, and the NaOH solution with a pH value of 8 is selected to prepare the aqueous phase solution in the experiment. 10-Hydroxycamptothecin is used as the water phase, and the oil phase is a mixture of liquid paraffin and sherwood oil with a volume ratio of 1: 2, and contains 4.0wt% emulsifier PO-500, wherein the volume ratio of the oil phase to the water phase is 30:1, take 2 mL of the water phase with a pH value of 8 and 60 mL of the oil phase to mix the pre-emulsion; then press the pre-emulsion repeatedly through the membrane pores under a pressure of 0.280 MPa until a W / O em...
Embodiment 2
[0063] In the experiment, a microporous membrane with a pore size of 2.8 μm was used to prepare drug-loaded gel microspheres. The specific implementation was as follows: the hydrophobic membrane with a pore size of 2.8 μm was soaked in a lipophilic substance, so that the porous membrane was fully wetted to ensure the drug-loaded gel microspheres on the membrane. The hydrophobic chain is completely stretched, and the NaOH solution with a pH value of 10 is used to prepare the aqueous phase solution in the experiment, as follows: a 1.0wt% alginate aqueous solution is prepared with a NaOH solution with a pH value of 10, and the concentration is 1mg / mL 10-Hydroxycamptothecin is used as the water phase, wherein the oil phase (O) is olive oil, and contains 2.0wt% emulsifier Arlacel83, and the volume ratio of the oil phase to the water phase is 40: 1; get the water phase with a pH value of 10 2mL was mixed with 80mL oil phase to prepare a pre-emulsion; then the pre-emulsion was repeate...
Embodiment 3
[0067]In the experiment, a microporous membrane with a pore size of 2.8 μm was used to prepare drug-loaded gel microspheres. The specific implementation was as follows: the hydrophobic membrane with a pore size of 2.8 μm was soaked in a lipophilic substance, so that the porous membrane was fully wetted to ensure the drug-loaded gel microspheres on the membrane. The hydrophobic chains are fully stretched. Prepare 0.5wt% alginate aqueous solution with pH=8 NaOH solution, and contain 10-hydroxycamptothecin at a concentration of 1mg / mL as the water phase, and the oil phase (O) is cottonseed oil, and contains 7.0wt% The emulsifier PO-310, the volume ratio of oil phase (O) and water phase (W) is 30: 1, get 2mL concentration and be that the alginate aqueous solution of 0.5wt% is mixed with 60mL oil phase to prepare pre-emulsion, then pre-emulsion The emulsion is repeatedly pressed through the membrane pores under a pressure of 0.300MPa until a W / O emulsion with uniform particle size ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com