Sorbent for endotoxins
An sorbent and endotoxin technology, which is applied in solid sorbent liquid separation, suction equipment, selective adsorption, etc., can solve the problems of complex production methods, high patient mortality, high production costs, etc. The effect of saving, improving the chance of survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0041] 1. Example 1: Production of sorbent (adsorbent) according to the present invention
[0042] In order to produce the sorbent according to the present invention, polymyxin B is used to coat neutral and hydrophobic polystyrene-divinylbenzene copolymers with different pore sizes, that is, polymyxin B via polystyrene -The hydrophobic interaction on the outer surface and the inner surface of the divinylbenzene copolymer is adsorbed.
[0043] 1.1. Provide neutral and hydrophobic polymers:
[0044] Table 1 lists the various average pore sizes of the polystyrene-divinylbenzene copolymer (referred to as "polymer" or "support" or "uncoated adsorbent" for short).
[0045] Table 1: Polystyrene-divinylbenzene copolymer
[0046] Name
Average pore size [nm]
#1822
15-20
#1823
15-20
#1824
30-40
#1825
80-100
#1826
80-100
[0047] The particle size of the polymer is 5μm + / -3-4μm.
[0048] 1.2. Coating polymyxin on polystyrene-divinylbenzene copolymer:
[0049] The polymers ...
example 2
[0055] 2. Example 2: Adsorption of endotoxin-batch test
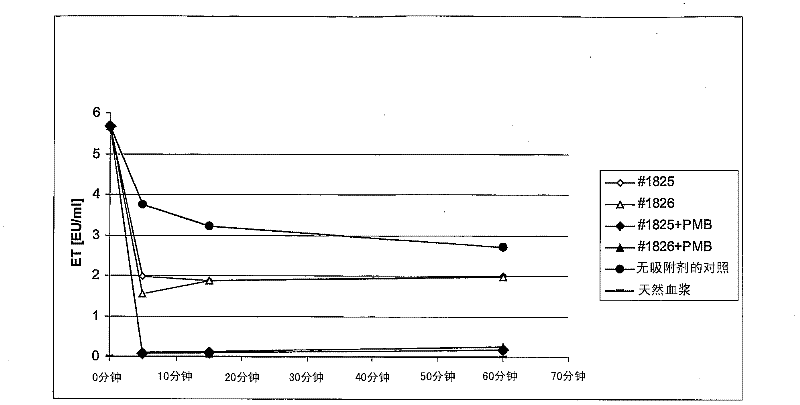
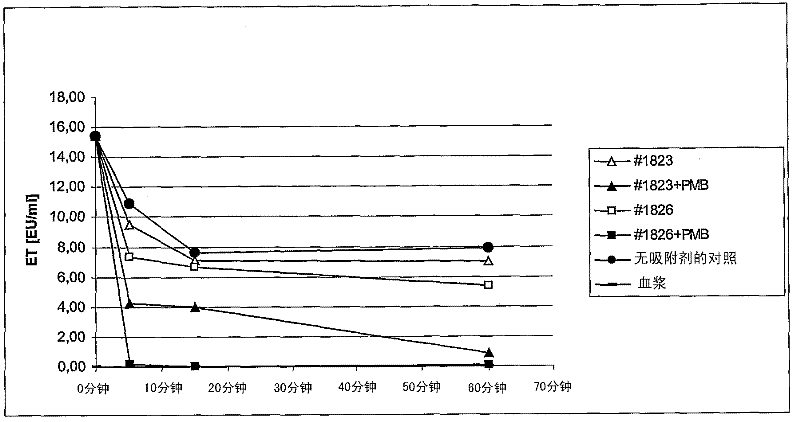
[0056] The polymer average pore size (PS) of #1825+PMB and #1826+PMB coated polymyxin B adsorbents is 80-100nm (see Example 1-Table 2), which is consistent with the corresponding #1825 and The #1826 uncoated adsorbent was compared for endotoxin binding.
[0057] 2.1. Preparation of adsorbent and adsorbent:
[0058] Prepare the following adsorbents (PS=80-100) according to the scheme of Example 1:
[0059] 1)#1825
[0060] 2)#1826
[0061] 3) #1825+PMB (PMB coated)
[0062] 4) #1826+PMB (PMB coated)
[0063] As described in 1.2, rinse the polymyxin B-coated and uncoated adsorbents 5 times with NaCl without a pyrogen. The 50% adsorbent suspension is finally produced in NaCl without a heat source.
[0065] Obtain 25ml heparin plasma from the donor (5x 9ml whole blood draw).
[0066] 2.3. Endotoxin solution:
[0067] LPS Pseudomonas aeruginosa (Sigma, L7018, lot number 109H4043). The stored in the -70℃ mi...
example 3
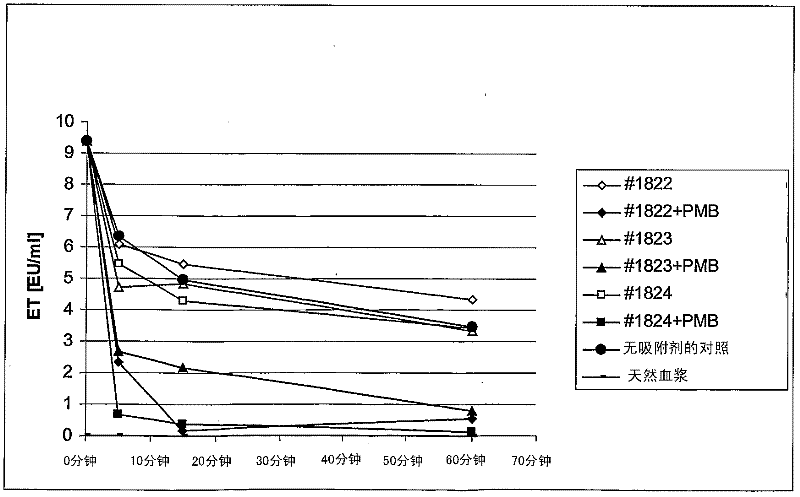
[0083] 3. Example 3: Adsorption of endotoxin-batch test
[0084] The average pore size (PS) is 15-20nm or 30-40nm coated polymyxin B adsorbent #1822+PMB, #1823+PMB and #1824+PMB and the corresponding uncoated adsorbent# 1822, #1823, and #1824 are compared for endotoxin binding.
[0085] 3.1. The adsorption body and the preparation of the adsorption body:
[0086] Prepare the following adsorbents according to the scheme of Example 1:
[0087] 1)#1822
[0088] 2)#1823
[0089] 2)#1824
[0090] 3) #1822+PMB (PMB coated)
[0091] 4) #1823+PMB (PMB coated)
[0092] 5) #1824+PMB (PMB coated)
[0093] As described in 1.2, rinse the polymyxin B-coated and uncoated adsorbents 5 times with NaCl without a pyrogen. The 50% adsorbent suspension is finally produced in NaCl without a heat source.
[0094] 3.2. Heparin plasma and endotoxin solution: Corresponds to parts 2.2 and 2.3.
[0095] 3.3. Test batch:
[0096] In the batch tested, the endotoxin solution was further diluted to a final concentration ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com