Preparation method of iodine removing agent
A technology for deiodination and silver oxidation, which is used in chemical instruments and methods, separation/purification of carboxylic acid compounds, organic chemistry, etc., and can solve problems such as waste water generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
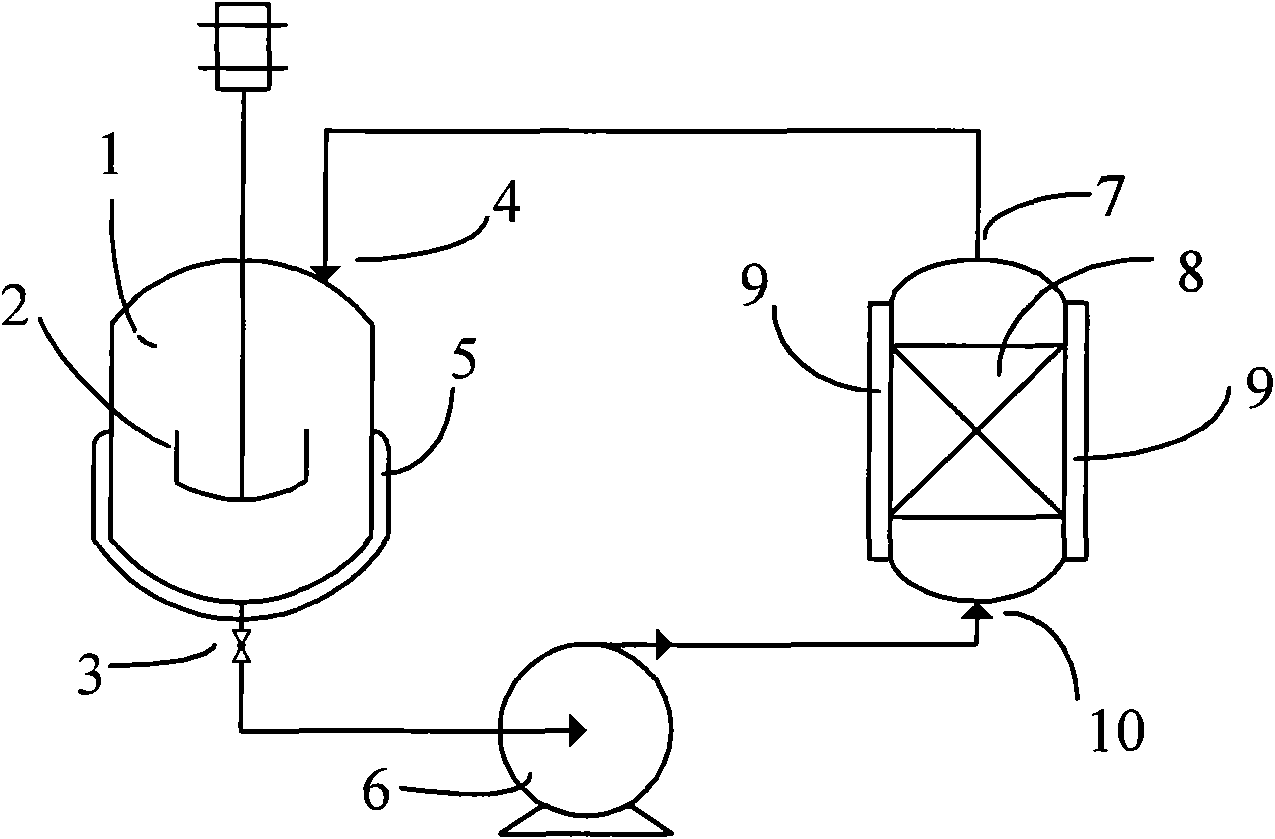
Image
Examples
Embodiment 1
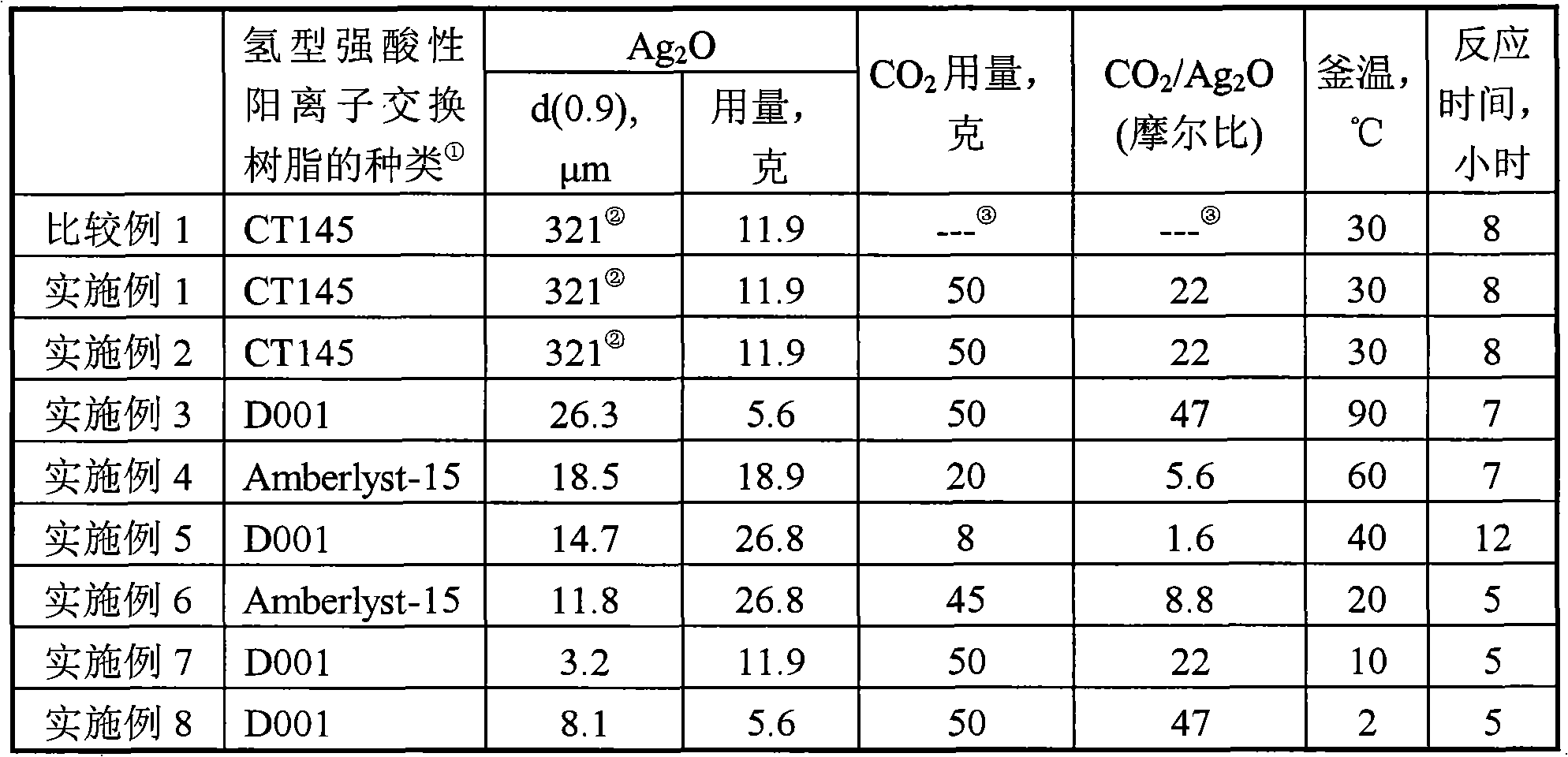
[0025] Add 1000 ml and 11.9 g of silver oxide (Ag 2 O) powder, stirred to Ag 2 O powder is evenly dispersed, add hydrogen-type CT145 cation exchange resin equivalent to 100 grams of dry weight, and then seal carbon dioxide gas (CO 2 ) 50 grams, maintain the temperature in the reactor at 30° C. and stir for 8 hours, slowly vent the pressure in the reactor to return to normal pressure, filter to obtain the deiodination agent, and produce 980 milliliters of filtrate. It should be emphasized that the filtration preparation process is not washed with water, and as [Example 2] proves, the water phase generated by filtration can be completely recycled, so there is no need to discharge waste water.
[0026] After testing, the silver content of the deiodination agent is 9.92%, and the iodine content in the effluent acetic acid is 4.6ppb after 4 hours of deiodination effect evaluation. The deiodination effect of the deiodination agent is equivalent to [Comparative Example].
[0027] ...
Embodiment 2
[0029] 980 milliliters of filtrates obtained after separating the deiodination agent in [Example 1] add 20 milliliters of water to make the liquid total amount reach 1000 milliliters and replace 1000 milliliters of water in [Example 1] as 1 cycle water and carry out 1 of the deiodination agent In the second cycle preparation experiment, the filtrate obtained in the first cycle test was also added to 1000 ml as the second cycle water to replace the water in [Example 1], and in this way until the 10th cycle preparation experiment was carried out. Other conditions in the cyclic preparation experiment were the same as in [Example 1]. This example is the result of the 10th cyclic preparation experiment.
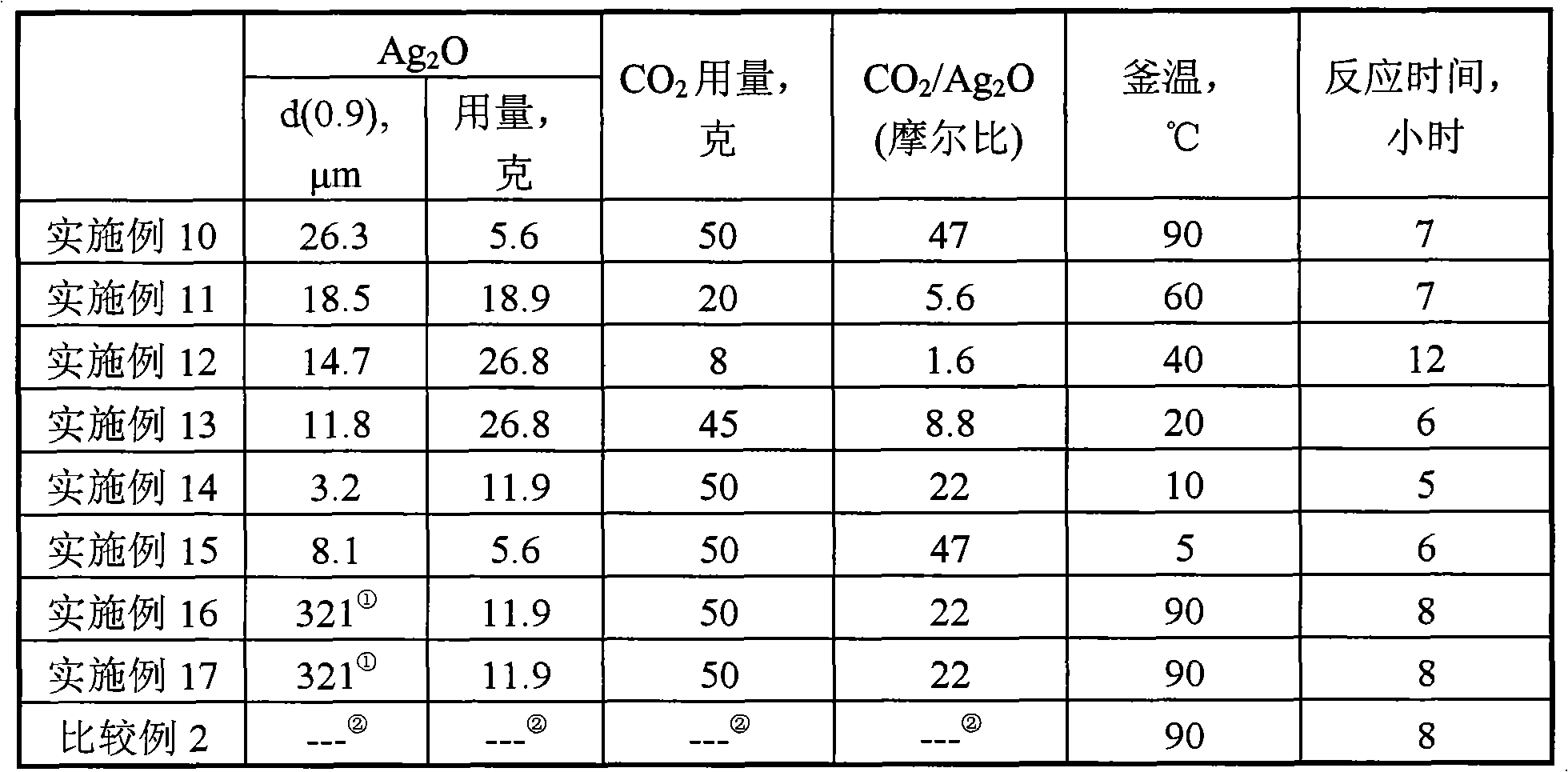
[0030] For the convenience of comparison, the important experimental conditions and results are listed in Table 1 and Table 2. Compared with the results of [Example 1], it can be seen that the filtrate produced in the preparation process is circulated for the preparation without a...
Embodiment 3
[0031] [Example 3] to [Example 8]
[0032] Except that the particle diameter of silver oxide, the consumption of silver oxide, the kind of hydrogen type strongly acidic cation exchange resin, the consumption of carbon dioxide, the temperature in the kettle and the reaction time may change, other operations are all the same as [Example 1]. For the convenience of comparison, the important experimental conditions and results are listed in Table 1 and Table 2. As demonstrated in [Example 2], the water phase obtained by filtration in [Example 3] to [Example 8] can also be recycled completely without discharging waste water.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| crystallinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com