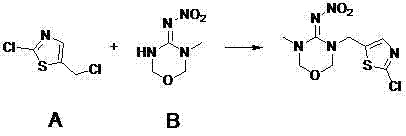
Preparation method for thiamethoxam
A technology of thiamethoxam and chloromethylthiazole, which is applied in the field of preparation of thiamethoxam, can solve the problems of thiamethoxam limiting the purity of compound A, weakening the stability of compound A, increasing the cost of final products, etc., and shortening the operation period. , The effect of reducing product cost and reducing the generation of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Take 18.5g of 3-methyl-4-nitroiminotetrahydro-1,3,5-oxadiazine and 16.8g of 2-chloro-5-chloromethylthiazole dissolved in 180g of acetone, add 0.4g of potassium iodide, 11g Potassium carbonate was stirred at room temperature for 8 hours, and the reaction was complete as detected by TLC. At this time, the system remained in a yellow suspension state. Evaporate the solvent acetone under reduced pressure at 40-50°C, add 400g of dichloromethane to the residue for soaking, filter to remove inorganic salts, collect the filtrate, the organic phase is a dark yellow transparent solution, and evaporate the dichloromethane to dryness at a temperature of 30-40°C to obtain crude Product, the crude product was heated to boiling and recrystallized with 130g of toluene, refluxed for 0.5h, cooled to room temperature to crystallize, and suction filtered to obtain 24.5g of beige thiamethoxam with a converted yield of 84% and a purity of 98%. The mother liquor was yellowish brown.
Embodiment 2
[0032] Take 18.5g of 3-methyl-4-nitroiminotetrahydro-1,3,5-oxadiazine and 16.8g of 2-chloro-5-chloromethylthiazole dissolved in 180g of acetonitrile, add 0.4g of potassium iodide, 11g Potassium carbonate was stirred under reflux for 12 hours. TLC detected that the reaction was complete. At this time, the system remained in a dark yellow suspension state. Evaporate the acetonitrile solvent under reduced pressure at 60-70°C, add 400g of dichloromethane to the residue for soaking, evaporate the dichloromethane to dryness at a temperature of 30-40°C to obtain a crude product, heat the crude product to boiling, recrystallize with 130g of toluene, and reflux After 0.5h, cool to room temperature and filter with suction to obtain 23.2 g of khaki-colored thiamethoxam, with a reduced yield of 78% and a purity of 96%.
Embodiment 3
[0034] According to 2006Mu00715, 18.5 g of 3-methyl-4-nitroiminotetrahydro-1,3,5-oxadiazine, 27.6 g of potassium carbonate were dissolved in 100 mL of DMF, and 16.8 g of 2-chloro-5-chloromethyl was added dropwise Base thiazole, control the temperature at 60-65 ° C, stir the reaction for 4 hours, the reaction system turns black completely. This system is highly alkaline, and a large amount of compound 2-chloro-5-chloromethylthiazole deteriorates during the reaction, and the color of thiamethoxam is very dark, and the yield is very low.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com