Method for modifying hollow glass microspheres by chemical precipitation process
A hollow glass microbead and chemical precipitation technology are used in the modification of hollow glass microbeads by chemical precipitation. problems, to achieve the effect of saving raw materials, excellent magnetic properties, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
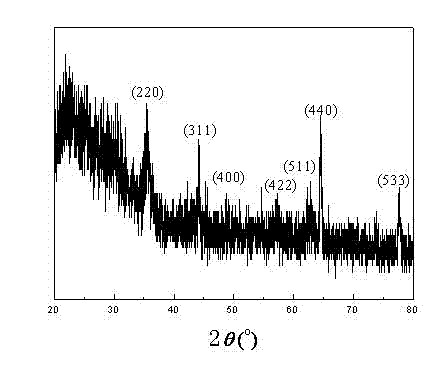
Image
Examples
Embodiment 1
[0027] Weigh 2 g of hollow glass microspheres, add them to 100 ml of oil-removing liquid, stir and react at 80°C for 20 minutes, then filter and wash with water, and dry the filtered hollow microspheres at 110°C for 1 hour. Weigh a certain amount of ferrous chloride and ferric chloride, dissolve them in deionized water, control the total iron concentration to 0.01mol / L, and the mass ratio of ferrous chloride to ferric chloride is 1:4, add pre- Treat the treated hollow glass microspheres at 10°C for 1 min, slowly add ammonia water with a concentration of 28% dropwise while stirring, so that the pH value of the solution is 5, and stir at a constant temperature of 100r / min for 1h. After the reaction is completed, The hollow microspheres were separated and filtered, washed with deionized water until neutral, and dried in a vacuum oven at 60°C for 30 minutes.
[0028] The saturation magnetization of the coated magnetic nano-ferric iron tetroxide hollow glass microspheres is 12emu / g...
Embodiment 2
[0030]Weigh 1 g of hollow glass microspheres, add them to 100 ml of oil-removing liquid, stir and react at 100°C for 30 minutes, then filter and wash with water, and dry the filtered hollow microspheres at 110°C for 2 hours. Weigh a certain amount of ferrous chloride and ferric chloride, dissolve them in deionized water, control the total iron concentration to 0.1mol / L, and the mass ratio of ferrous chloride to ferric chloride is 4:1, add pre- Treat the treated hollow glass microspheres at 50°C for 10 minutes, slowly add ammonia water with a concentration of 28% while stirring, so that the pH of the solution is 11, treat at a constant temperature of 900r / min for 3 hours, and separate after the reaction is completed. The microbeads were filtered out, washed with deionized water until neutral, and dried in a vacuum oven at 60°C for 60 min.
[0031] The saturation magnetization of the coated magnetic nano-ferric iron tetroxide hollow glass microspheres is 45 emu / g, and the residu...
Embodiment 3
[0033] Weigh 1.5 g of hollow glass microspheres, add them to 100 ml of oil-removing liquid, stir and react at 95°C for 25 minutes, then filter and wash with water, and dry the filtered hollow microspheres at 110°C for 1.5h. Weigh a certain amount of ferrous chloride and ferric chloride, dissolve them in deionized water, control the total iron concentration to 0.05mol / L, and the mass ratio of ferrous chloride to ferric chloride is 2:1, add pre- Treat the treated hollow glass microspheres at 30°C for 5 minutes, slowly add ammonia water with a concentration of 28% dropwise while stirring, so that the pH value of the solution is 9, treat at a constant temperature of 500r / min for 2 hours, and separate after the reaction is completed. The hollow microspheres were filtered out, washed with deionized water until neutral, and dried in a vacuum oven at 60°C for 40 minutes.
[0034] The saturation magnetization of the coated magnetic nano-ferric iron tetroxide hollow glass microspheres i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Saturation magnetization | aaaaa | aaaaa |
| Saturation magnetization | aaaaa | aaaaa |
| Saturation magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com