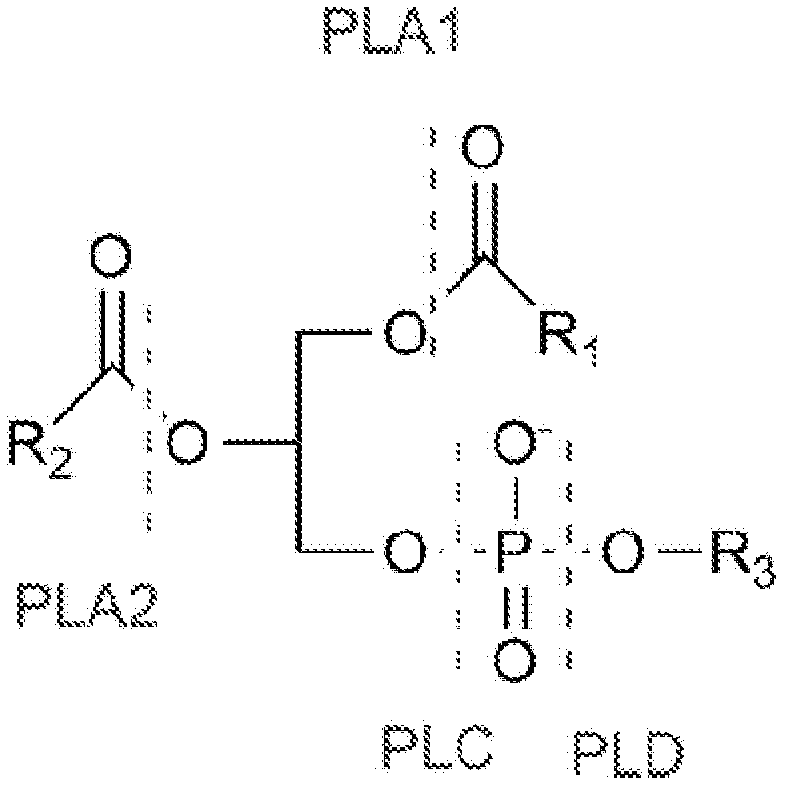
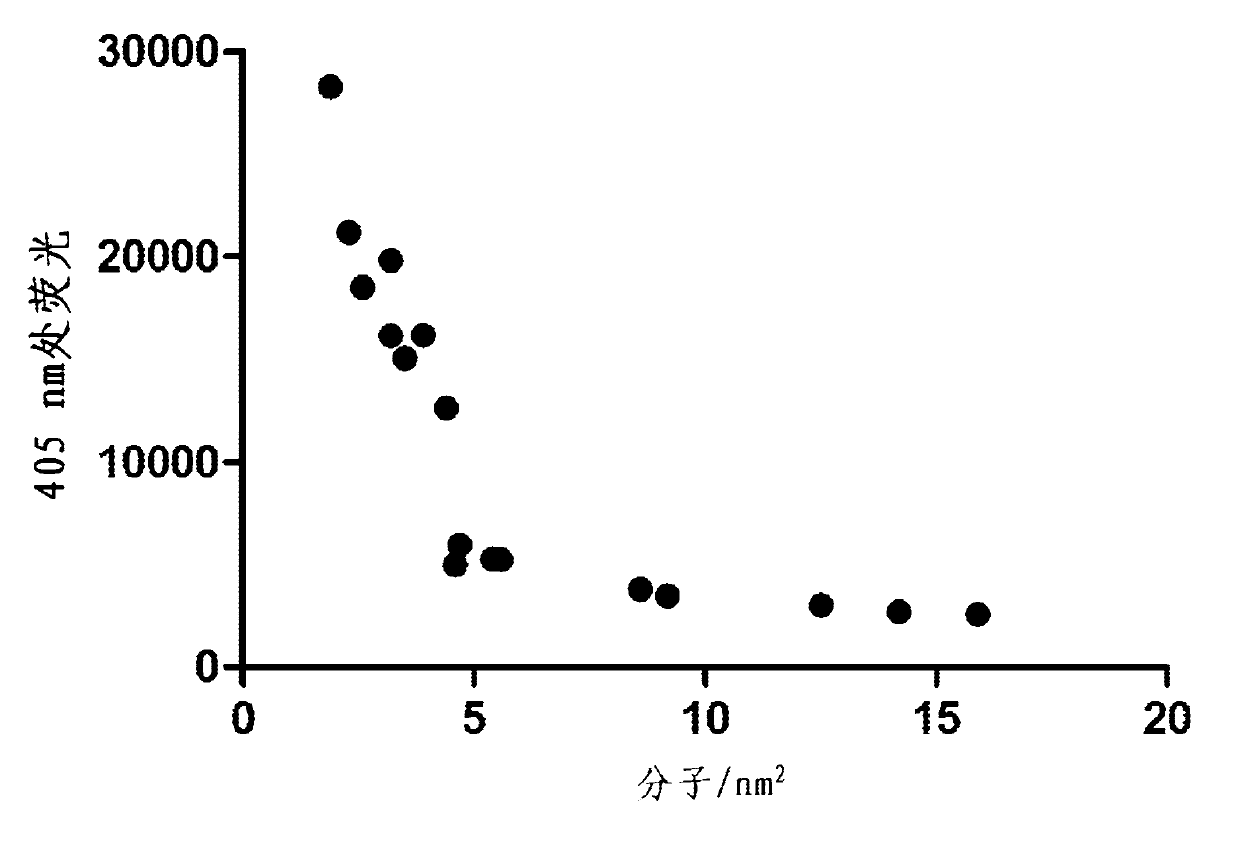
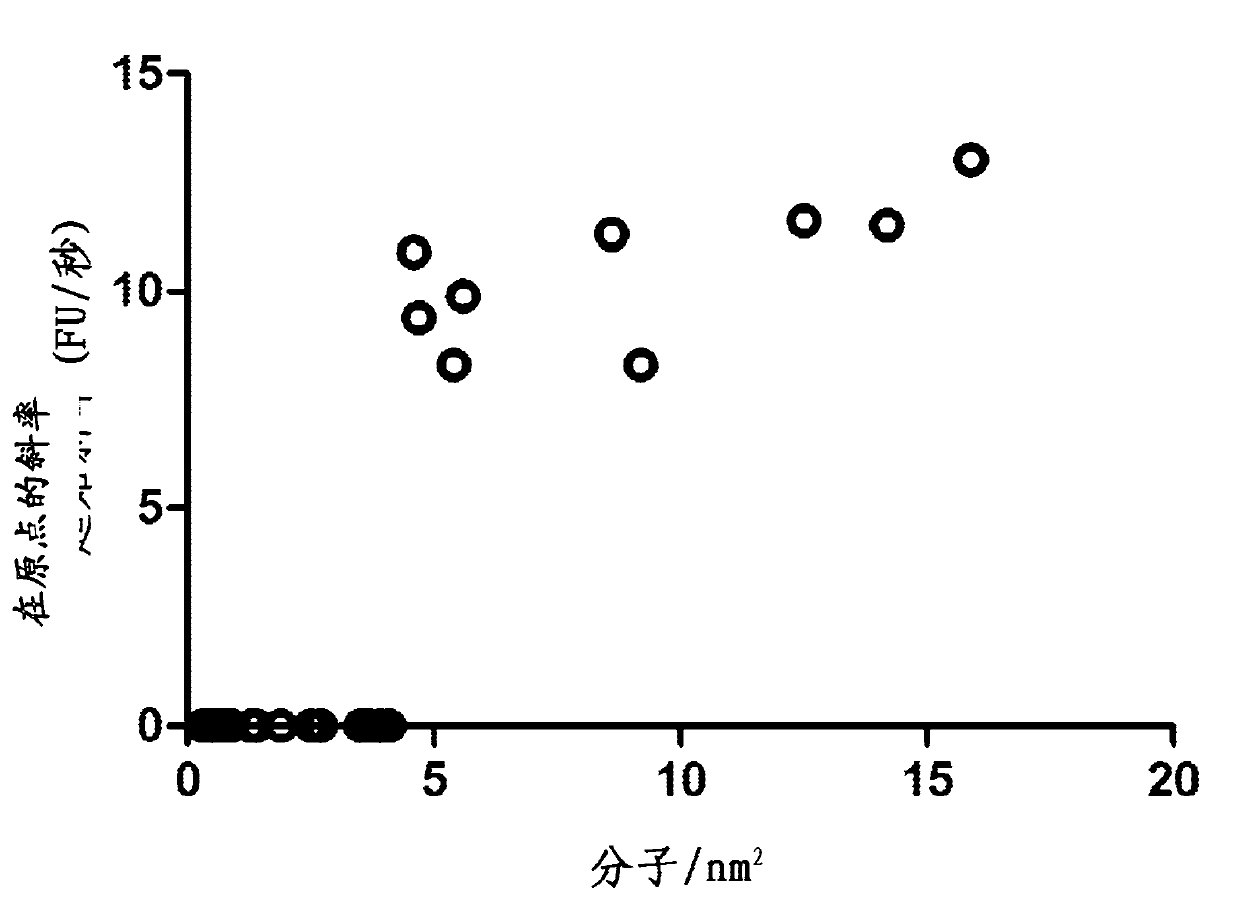
Enzymatic assay for the quantitative determination of phospholipase A1 or A2 activity in a sample
A technology of phospholipase and activity, applied in the field of enzyme assay for quantitative determination of phospholipase activity in samples, can solve the problem of low coverage of fluorescent phospholipid molecules, radioactive labeling is not suitable for diagnostic applications, and molecular/nm2 packing density is not suitable for fluorescence assays And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0213] 1-Preparation of the phospholipid-coated solid phase according to the inventive method
[0214] Materials and Reagents
[0215] β-py-C 10 -glycerophosphate (β-py-C 10 -PG) was from Molecular Probes / Invitrogen. [mu]m polystyrene microspheres (beads) were purchased from Merck / Estapor. 96-well plates were purchased from Nunc. All other reagents were from Sigma-Aldricht.
[0216] Experimental program
[0217] All steps are performed in glassware not directly exposed to light.
[0218] [beta]-py-C10-Glycerol phosphate was dissolved in 10% methanol / 90% chloroform (v / v) in a glass vessel using ultra-sonication. The exact molar concentration of β-py-C10-phosphoglycerol was determined by means of a standard curve prepared by means of a five-point standard curve (1 / 100 and 1 / 1600 dilution in methanol , prepared from the absorbance at 342 nm of two-fold serial dilutions), and the blank was defined as a 1 / 100 dilution of 10% methanol / 90% chloroform (v / v) solution in me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com