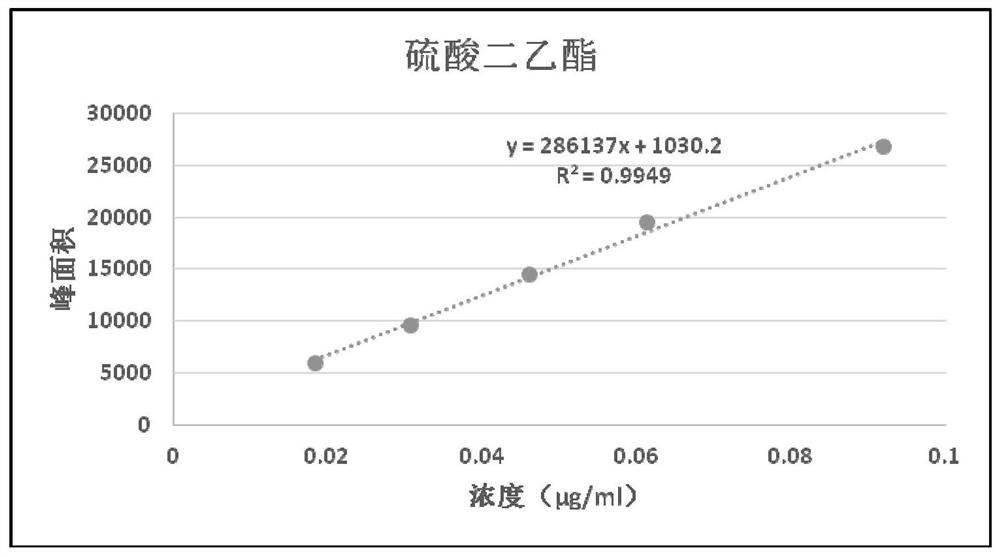
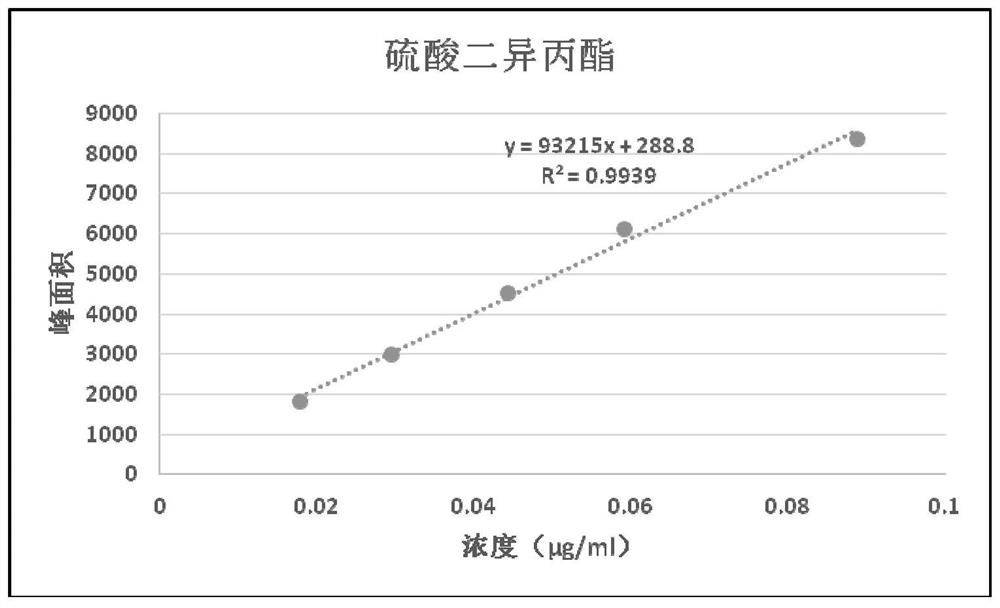
Method for detecting genotoxic impurities of diethyl sulfate and diisopropyl sulfate in calcium dobesilate capsule
A technology of diisopropyl sulfate and calcium dobesilate, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of DNA mutagenesis and damage, and achieve the effect of excellent evaluation index and simple operation of derivatization treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 of the present invention: The present invention is described below by examples, but the present invention is not limited thereto.
[0028] The methods used in the following examples are conventional methods unless otherwise specified; the reagents and materials used in the following implementations can be obtained from commercial sources unless otherwise specified.
[0029] 1. Instruments and reagents
[0030] Agilent 7890A-5975C gas chromatography-tandem mass spectrometer, Mettler Toledo XP205 electronic balance, Agilent DB-624UI chromatographic column, Shanghai Hetai Medium S800UVT ultrapure water instrument, Merck HPLC grade acetonitrile, MACKLIN 99% thiosulfuric acid pentahydrate Sodium, MACKLIN 99.5% sodium iodide, Aladdin diethyl sulfate 99.0%, TOKYO CHEMICAL INDUSTRY CO., LTD. Diisopropyl sulfate 97.0%.
[0031] Derivatization reagent preparation method: Weigh about 60g of sodium iodide and 50mg of sodium thiosulfate pentahydrate into a 100ml beaker...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com