Method for synthesizing analgin
A technology of analgin and methylamphenicol, which is applied in the field of compound synthesis, can solve the problems of long synthesis route, many by-products and high cost, and achieve the effects of short production cycle, pollution reduction and quality assurance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
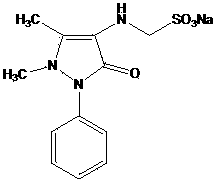
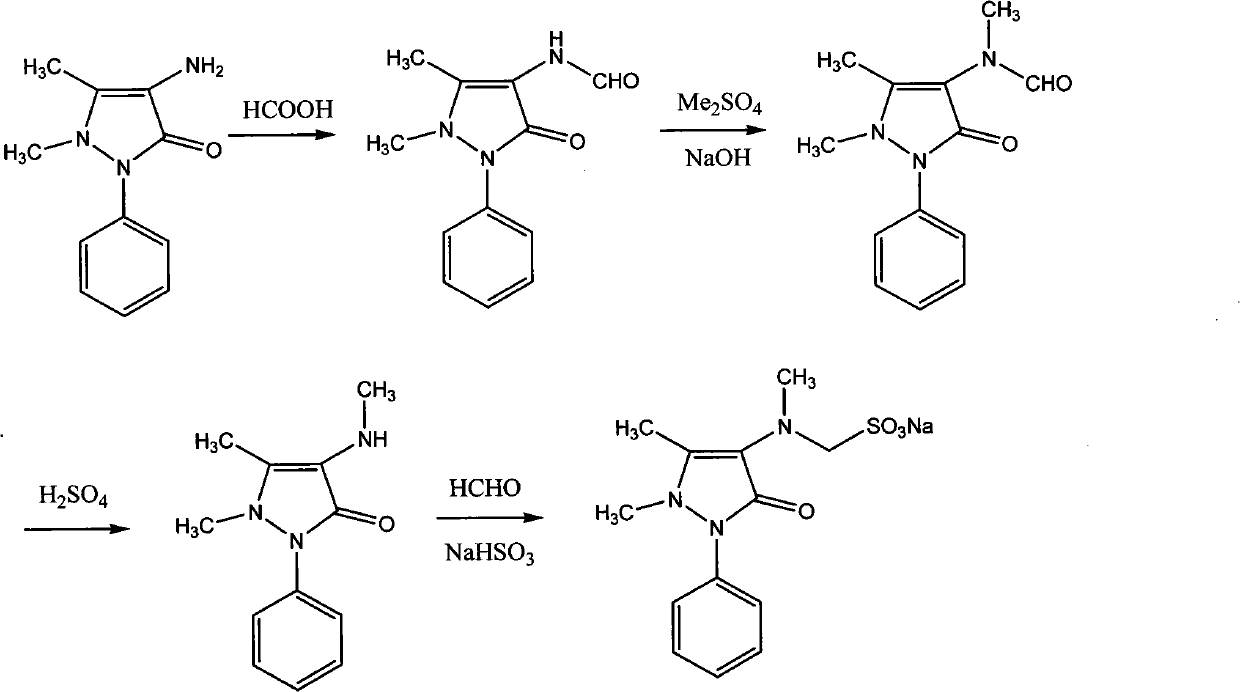
[0047] Example 1 Preparation of 4-N-desmethyl Sulpyrine
[0048] 20.3 g of 4-aminoantipyrine (AA) (0.1 mol), 10.4 g of sodium bisulfite (0.1 mol), 3 g of paraformaldehyde (0.1 mol) and 100 ml of 95% ethanol were added to a 250 ml reaction bottle, stirred and heated to reflux for 1 hour. After the reaction liquid was lowered to 45°C, solid 4-N-desmethyl sulpyrine was added to the reaction liquid to cause crystallization. After vigorously stirring for half an hour, the solid was collected by filtration to obtain 30.3 g of 4-N-desmethyl sulpyrine. The rate is 90%; melting point: 217°C; 1 H NMR (400MHz, DMSO-d 6 )δ: 2.18(s, 3H), 2.80(s, 3H), 3.78(d, J=6.96Hz, 2H), 4.00(m, 1H), 7.25(m, 1H), 7.45(m, 4H); 13 C NMR (100MHz, DMSO-d 6 )δ: 161.8, 140.8, 135.4, 128.9(2), 125.3, 122.1(2), 120.0, 62.4, 37.7, 10.4.
Embodiment 2
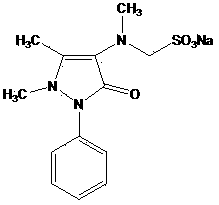
[0049] Embodiment 2 Preparation of Sulpyrine
[0050] 33 grams of 4-N-desmethyl sulpyrine (0.1mol) were dissolved in 100 milliliters of 95% ethanol, and 16.5 grams of dimethyl sulfate (0.13mol) and 10.4 grams of sodium bicarbonate (0.13mol) were added successively, and stirred And heated to 60 ° C for 1 hour. The reaction solution was neutralized with acetic acid to pH 7-8, stirred and cooled, and then crystallized. The solid was collected by filtration to obtain 31 g of Sulpyrine with a yield of 89%; the purity determined by HPLC was 99.5%.
Embodiment 3
[0051] Embodiment 3 The preparation of Sulpyrine (one-pot method)
[0052] 20.3 g of 4-aminoantipyrine (AA) (0.1 mol), 10.4 g of sodium bisulfite (0.1 mol), 3 g of paraformaldehyde (0.1 mol) and 100 ml of 95% ethanol were added to a 250 ml reaction bottle, stirred and heated to reflux for 1 hour. The reaction solution was stirred and cooled to 40° C., then 16.5 g of dimethyl sulfate (0.13 mol) and 10.4 g of sodium bicarbonate (0.13 mol) were sequentially added, stirred and heated to 60° C. for 1 hour. The reaction solution was neutralized with acetic acid to pH 7-8, stirred and cooled, and then crystallized. The solid was collected by filtration to obtain 26 g of Sulpyrine with a yield of 74%. The purity determined by HPLC was 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com