Preparation method of macroporous large specific surface magnetic photocatalyst Fe3O4/TiO2
A high specific surface, visible light technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve difficulties and other problems, and achieve the effect of simple method, rapid solid-liquid separation, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
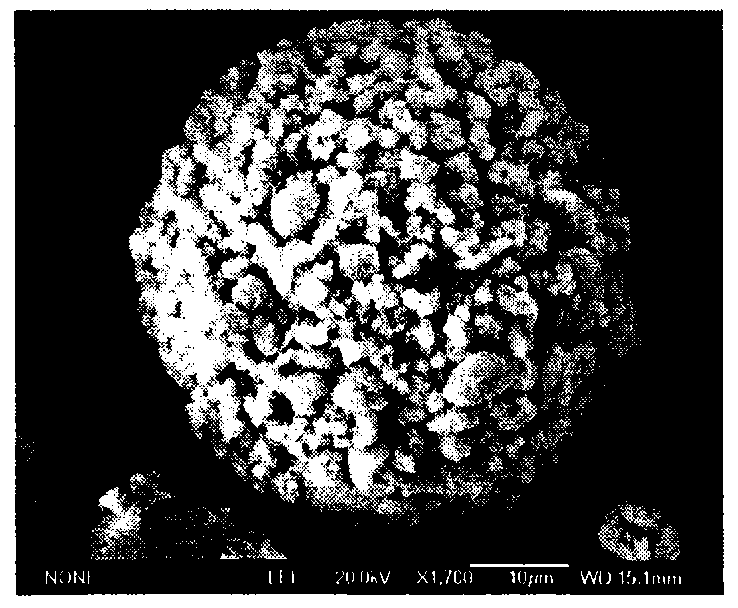
Image
Examples
Embodiment 2
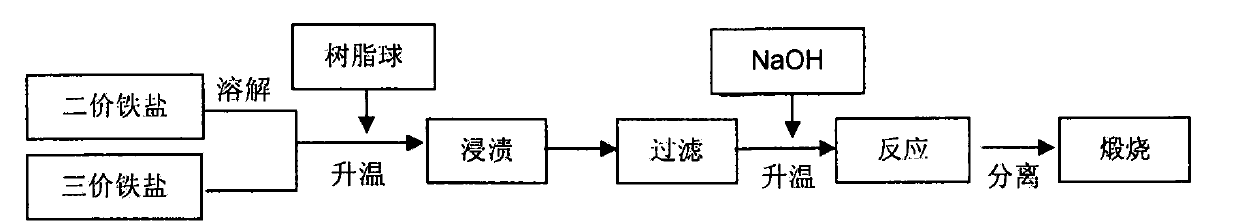
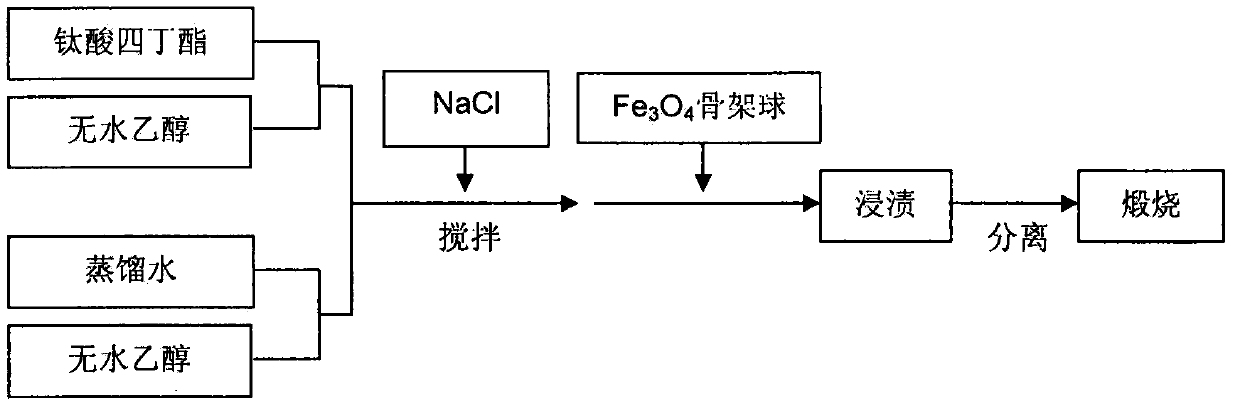
[0025] Fe(NO 3 ) 2 ·6H 2 O and Fe 2 (SO 4 ) 3 9H 2 O is dissolved in 50mL distilled water according to the iron ion molar ratio of 1:2, and is prepared into an aqueous solution with a total iron ion molar fraction of 1.8%, and is added to the 2In the four-necked bottle with the device and thermometer, start stirring and add 10 parts of macroporous cross-linked polystyrene microspheres to it, the system is heated up to 45°C, and the temperature is maintained for 4h, then poured out, filtered and collected. Add polystyrene microspheres into a 100mL Erlenmeyer flask with a stopper, and put the flask into a constant temperature water bath. When the temperature rises to 80°C, add 5 parts of sodium hydroxide to it, shake gently for 1min and then keep the temperature After reacting for 50min and cooling to room temperature, Fe was obtained by magnetic separation 3 o 4 / polystyrene microspheres, put them into a muffle furnace, calcinate at 600°C for 5h in a nitrogen atmosphere...
Embodiment 4
[0029] FeCl 2 4H 2 O and Fe(NO 3 ) 3 9H 2 O is dissolved in 50mL distilled water according to the iron ion molar ratio of 1:2, and is prepared into an aqueous solution with a total iron ion molar fraction of 1.9%, and is added to the 2 In the four-necked bottle of the device and thermometer, start stirring and add 8 parts of macroporous cross-linked polydivinylbenzene microspheres to it, the system is heated up to 55°C, and the temperature is maintained for 3h, then poured out, filtered and collected. Pore cross-linked polydivinylbenzene microspheres were added to a 100mL Erlenmeyer flask with a stopper, and the flask was placed in a constant temperature water bath. When the temperature rose to 65°C, 4 parts of sodium hydroxide was added to it, and gently Shake for 1 min, then react at constant temperature for 1 h, cool to room temperature, and obtain Fe by magnetic separation 3 o 4 / polydivinylbenzene microspheres, put them into a muffle furnace, calcinate at 800°C fo...
Embodiment 5
[0031] FeSO 4 ·7H 2 O and FeCl 3 ·6H 2 O is dissolved in 50mL distilled water according to the iron ion molar ratio of 1:2, and is prepared into an aqueous solution with a total iron ion molar fraction of 3%, and is added to the 2 In the four-necked bottle with the device and thermometer, start stirring and add 7 parts of macroporous cross-linked polyacrylonitrile microspheres to it, the system is heated up to 45°C, keep this temperature for 5h, pour out, filter and collect the impregnated macroporous cross-linked polyacrylonitrile microspheres. Add polyacrylonitrile microspheres into a 100mL Erlenmeyer flask with a stopper, and put the flask into a constant temperature water bath. When the temperature rises to 70°C, add 5 parts of sodium hydroxide to it, shake gently for 1min and then keep the temperature After reacting for 45min and cooling to room temperature, Fe was obtained by magnetic separation 3 o 4 / polyacrylonitrile microspheres, put them into a muffle furnace, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com