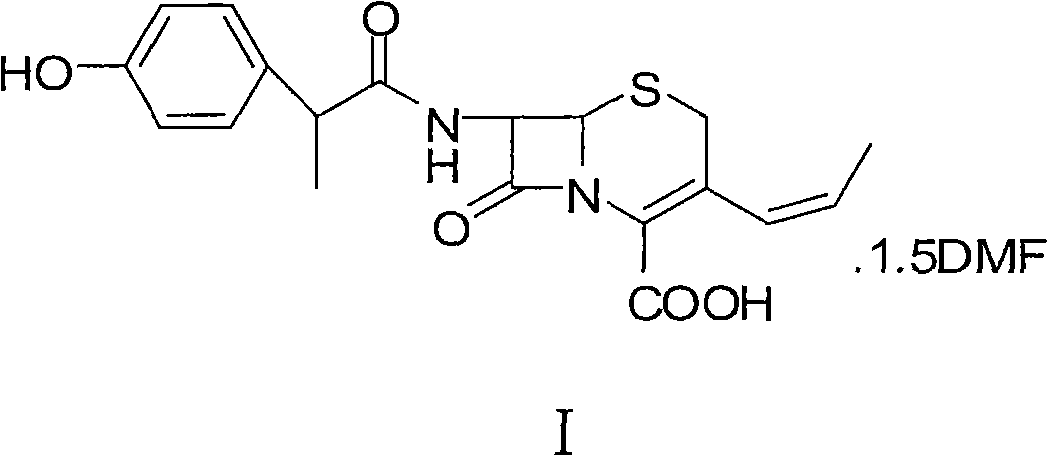
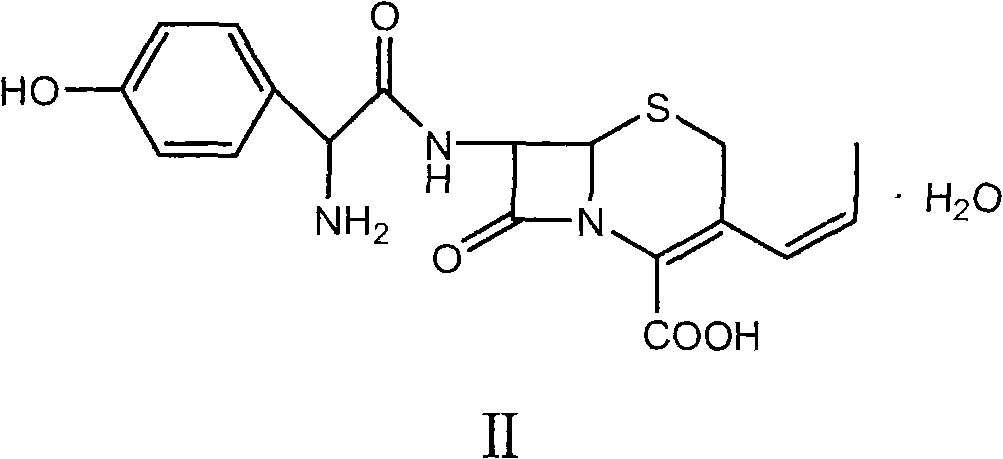
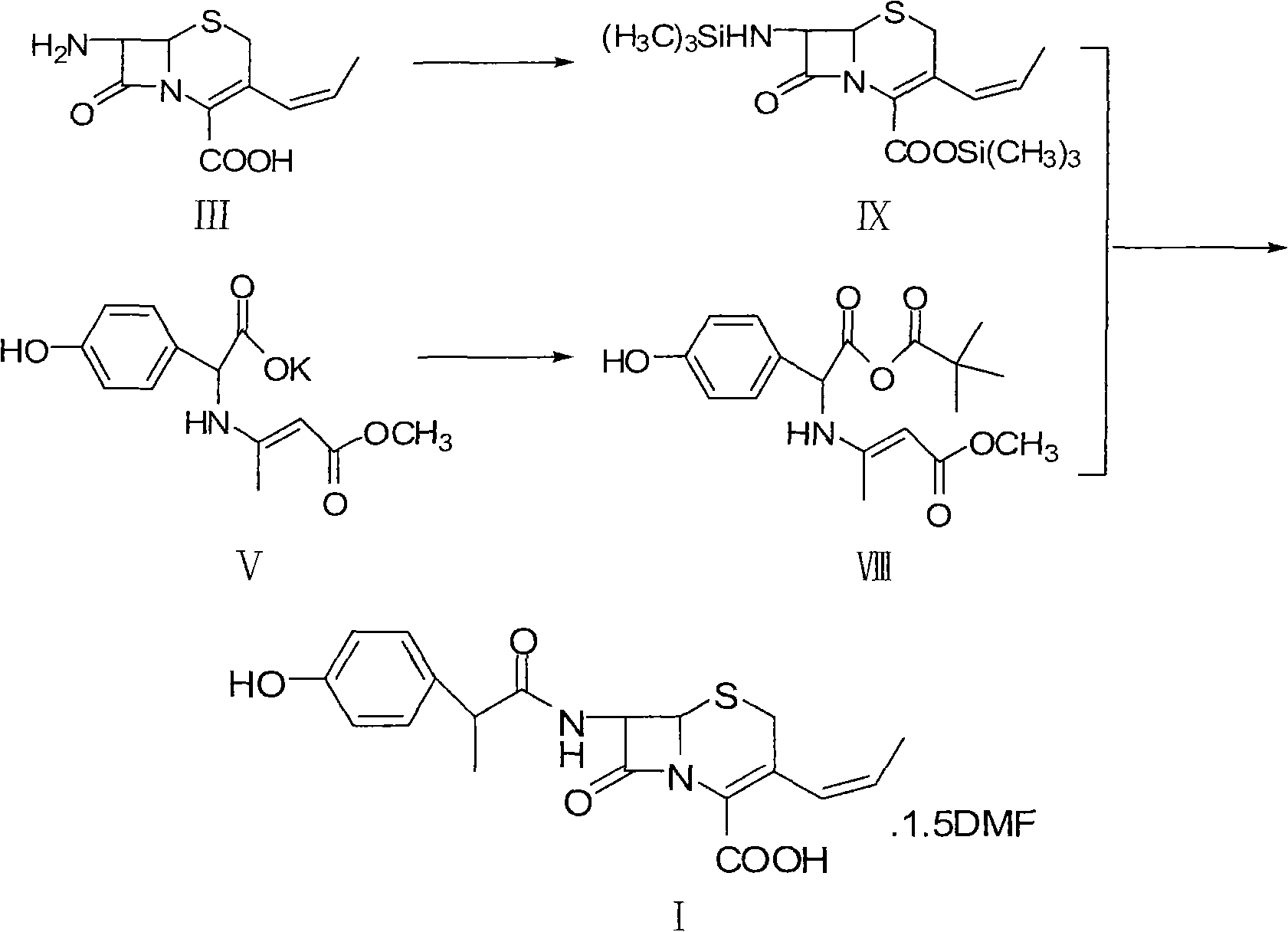
Method for preparing cefprozil dimethyl formamide solvate
A technology of propylene dimethyl formamide and solvate, which is applied in the field of preparation of important intermediates of antibiotics, and can solve the problems of high price and unstable silylating reagents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of cefprozil DMF solvate (I)
[0042] (1) Synthesis of 7-APCA-tetramethylguanidine salt (compound VII)
[0043] 7-APCA (31.155 g, 0.1298 mol) was suspended in dichloromethane (240 mL), and cooled to -15~-5°C. Tetramethylguanidine (14.928 g, 0.1298 mol) was added dropwise, and the mixture was kept warm and stirred for 30 minutes, and kept warm for later use.
[0044] (2) Synthesis of mixed anhydride (compound VIII)
[0045] Add D-p-hydroxyphenylglycine potassium salt (45.36 g, 0.1497 mol) into a mixed solution of dichloromethane (60 mL), DMF (120 mL) and pyridine (0.39 mL), and stir at room temperature to obtain a suspension. When the temperature was lowered to -35°C, a mixed solution of pivaloyl chloride (18.63 mL) and dichloromethane (60 mL) was added dropwise. After the dropwise addition, continue to stir at the same temperature for 3h.
[0046] (3) Condensation reaction
[0047] Add the 7-APCA-tetramethylguanidine salt prepared in s...
Embodiment 2
[0050] Embodiment 2: the preparation of cefprozil DMF solvate (I)
[0051] (1) Synthesis of 7-APCA-tetramethylguanidine salt (compound VII)
[0052] 7-APCA(III) (31.155 g, 0.1298 mol) was suspended in dichloromethane (240 mL), and cooled to -15~-5°C. Tetramethylguanidine (14.928 g, 0.1298 mol) was added dropwise, and the mixture was kept warm and stirred for 30 minutes, and kept warm for later use.
[0053] (2) Synthesis of mixed anhydride (compound VIII)
[0054] Add D-p-hydroxyphenylglycine potassium salt (45.36g, 0.1497mol) into a mixed solution of dichloromethane (117mL), DMF (74.55mL) and pyridine (0.39mL), and stir at room temperature to obtain a suspension. When the temperature was lowered to -35°C, 18.63 mL of pivaloyl chloride was added dropwise. After the dropwise addition, continue to stir at the same temperature for 2 h, and finally add 62 mL of cold DMF to dilute.
[0055] (3) Condensation reaction
[0056] Add the 7-APCA-tetramethylguanidine salt prepared in s...
Embodiment 3
[0059] Embodiment 3: the preparation of cefprozil DMF solvate (I)
[0060] (1) Synthesis of 7-APCA-tetramethylguanidine salt (compound VII)
[0061] 7-APCA(III) (31.155 g, 0.1298 mol) was suspended in dichloromethane (240 mL), and cooled to -15~-5°C. Tetramethylguanidine (14.928 g, 0.1298 mol) was added dropwise, and the mixture was kept warm and stirred for 30 minutes, and kept warm for later use.
[0062] (2) Synthesis of mixed anhydride (compound VIII)
[0063] Add D-p-hydroxyphenylglycine potassium salt (45.36g, 0.1497mol) into a mixed solution of dichloromethane (117mL), DMF (74.55mL) and pyridine (0.39mL), and stir at room temperature to obtain a suspension. When the temperature was lowered to -35°C, 18.63 mL of pivaloyl chloride was added dropwise. After the dropwise addition, continue to stir at the same temperature for 2 h, and finally add 62 mL of cold DMF to dilute.
[0064] (3) Condensation reaction
[0065] Add the 7-APCA-tetramethylguanidine salt prepared in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com