Preparation method for nucleic acid quality control by chimeric exogenous independent sequence
A sequence and exogenous technology, applied in biochemical equipment and methods, DNA preparation, microbial measurement/inspection, etc., can solve the problems of low identification accuracy and complex identification reaction, and achieve good reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
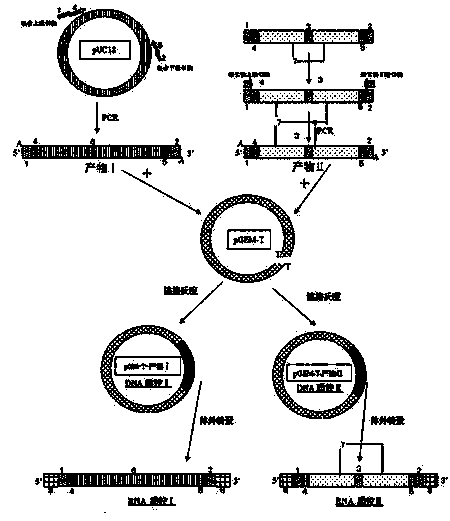
[0023] Example 1, see figure 1 Schematic diagram of the preparation of nucleic acid quality control of chimeric exogenous unrelated sequences, when the detection reaction is based on the length of the product, use method I; when method I cannot meet the actual needs, especially the detection reaction is taqman real-time quantitative PCR or similar probes in the middle When detecting the reaction, use method II.
[0024] (1) Selection of irrelevant sequence sources
[0025] The method I: the irrelevant sequence comes from the existing plasmid vector, such as pUC18, pET30 and so on. Select a DNA sequence from an existing plasmid vector, and use biological software to analyze the GC content and secondary conformation of the DNA sequence. The DNA sequence that meets the following conditions is selected as the irrelevant sequence I: a) The irrelevant sequence I is compatible with the microorganisms to be detected. The homology is less than 5%, b) the GC content is between 45% and...
Embodiment 2
[0039] Example 2, identification of quality: Both the DNA quality control and the RNA quality control can measure OD260, OD260 / OD280 ratio by spectrophotometer and agarose electrophoresis to determine their concentration. It is also necessary to use PCR and RT-PCR to identify the validity of the quality control nucleic acid respectively. Good-quality quality control DNA and RNA should have clear electrophoresis bands, OD260 / OD280 between 1.8 and 2.0, and the amplification curve of the detection reaction is normal.
Embodiment 3
[0040] Embodiment 3, the application of quality control
[0041] (1) Quality control PCR / reverse transcription PCR detection reaction
[0042] DNA quality control and RNA quality control respectively quality control PCR / reverse transcription PCR (RT-PCR) detection reaction. Specifically, for any molecular diagnostic kit, the DNA quality control and the RNA quality control can be used as samples to be tested for the detection reaction of the kit, respectively, and the detection reaction can be carried out according to the instructions of the kit. If the quality control sample has a positive test result, it indicates that the kit is well preserved and the test reaction is established; if the quality control sample shows a negative test result, it indicates that the kit reagents are invalid or the test personnel have performed poorly, and the test reaction has failed.
[0043] (2) Differentiate between true positive amplification and false positive amplification
[0044] If the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com