One-pot method for preparing crucial intermediate in oseltamivirphosphate synthesizing reaction
A technology for synthesis reaction and intermediate, which is applied in the field of obtaining key intermediates of Tamiflu synthesis reaction by "one-pot method", can solve the problems of low final yield, long synthesis route, difficult purification, etc., and achieves simple separation and purification and synthesis. The effect of shortened cycle and short reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0006] Example 1: "One-pot method" to obtain the key intermediate of Tamiflu synthesis reaction
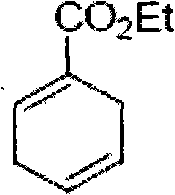
[0007] Step 1: Synthesis of ethyl 1-formate-1,4-diene cyclohexane, the chemical formula is as follows
[0008]
[0009] Seal the 1,3-butadiene in the bottle and cool it to -78°C, then directly pour it into a 40ml high-pressure glass flask pre-cooled at -78°C, add 20g of ethyl ethyl propiolate, 2g of hydroquinone and a Magnetic stir bar. The reaction flask was sealed and stirred at 110° C. for 3 days (note: a protective pad should be used when sealing, which is convenient for monitoring the reaction). The reaction mixture should be cooled to -78°C before opening and sealing, and the contents of the bottle are distilled under reduced pressure (benzyl alcohol 90-92°C / 8mm) to obtain clean oily ethyl 1-formate-1,4-dienecyclohexyl The alkane product (26.4 g, 85% yield). 1 H NMR (500MHz, CDCl 3 )δ6.97(m, 1H), 5.78(m, 1H), 5.67(m, 1H), 4.22(q, J=7.0, 2H), 2.90(m, 1H), 1.31(t, J=7.0...
Embodiment 2
[0018] Example 2: "One pot method" to obtain the key intermediate of Tamiflu synthesis reaction
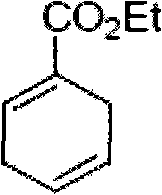
[0019] Step 1: Synthesis of ethyl 1-formate-1,4-diene cyclohexane, the chemical formula is as follows
[0020]
[0021] Seal the 1,3-butadiene in the bottle and cool it to -78°C, then directly pour it into a 40ml high-pressure glass flask pre-cooled at -78°C, add 20g of ethyl ethyl propiolate, 2g of hydroquinone and a Magnetic stir bar. The reaction flask was sealed and stirred at 110° C. for 3 days (note: a protective pad should be used when sealing, which is convenient for monitoring the reaction). The reaction mixture should be cooled to -78°C before opening and sealing, and the contents of the bottle are distilled under reduced pressure (benzyl alcohol 90-92°C / 8mm) to obtain clean oily ethyl 1-formate-1,4-dienecyclohexyl The alkane product (26.4 g, 85% yield). 1 H NMR (500MHz, CDCl 3)δ6.97(m, 1H), 5.78(m, 1H), 5.67(m, 1H), 4.22(q, J=7.0, 2H), 2.90(m, 1H), 1.31(t, J=7.0,...
Embodiment 3
[0030] Example 3: "One pot method" to obtain the key intermediate of Tamiflu synthesis reaction
[0031] Step 1: Synthesis of ethyl 1-formate-1,4-diene cyclohexane, the chemical formula is as follows
[0032]
[0033] Seal the 1,3-butadiene in the bottle and cool it to -78°C, then directly pour it into a 40ml high-pressure glass flask pre-cooled at -78°C, add 20g of ethyl ethyl propiolate, 2g of hydroquinone and a Magnetic stir bar. The reaction flask was sealed and stirred at 110° C. for 3 days (note: a protective pad should be used when sealing, which is convenient for monitoring the reaction). The reaction mixture should be cooled to -78°C before opening and sealing, and the contents of the bottle are distilled under reduced pressure (benzyl alcohol 90-92°C / 8mm) to obtain clean oily ethyl 1-formate-1,4-dienecyclohexyl The alkane product (26.4 g, 85% yield). 1 H NMR (500MHz, CDCl 3 )δ6.97(m, 1H), 5.78(m, 1H), 5.67(m, 1H), 4.22(q, J=7.0, 2H), 2.90(m, 1H), 1.31(t, J=7.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com