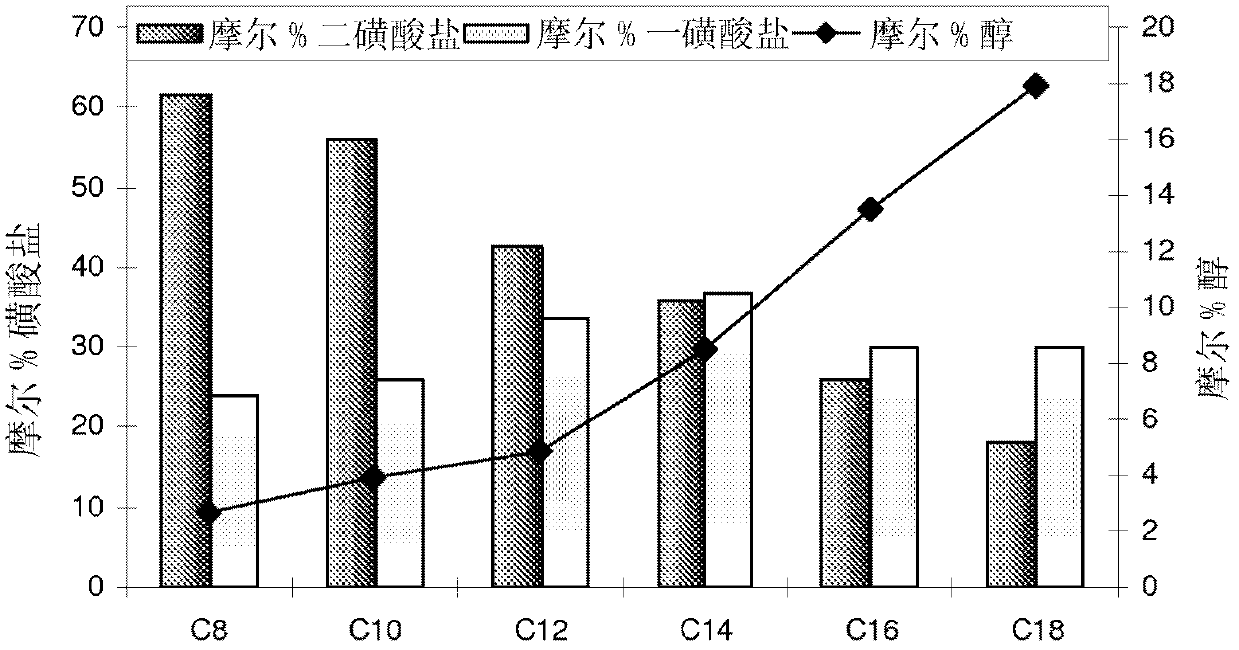
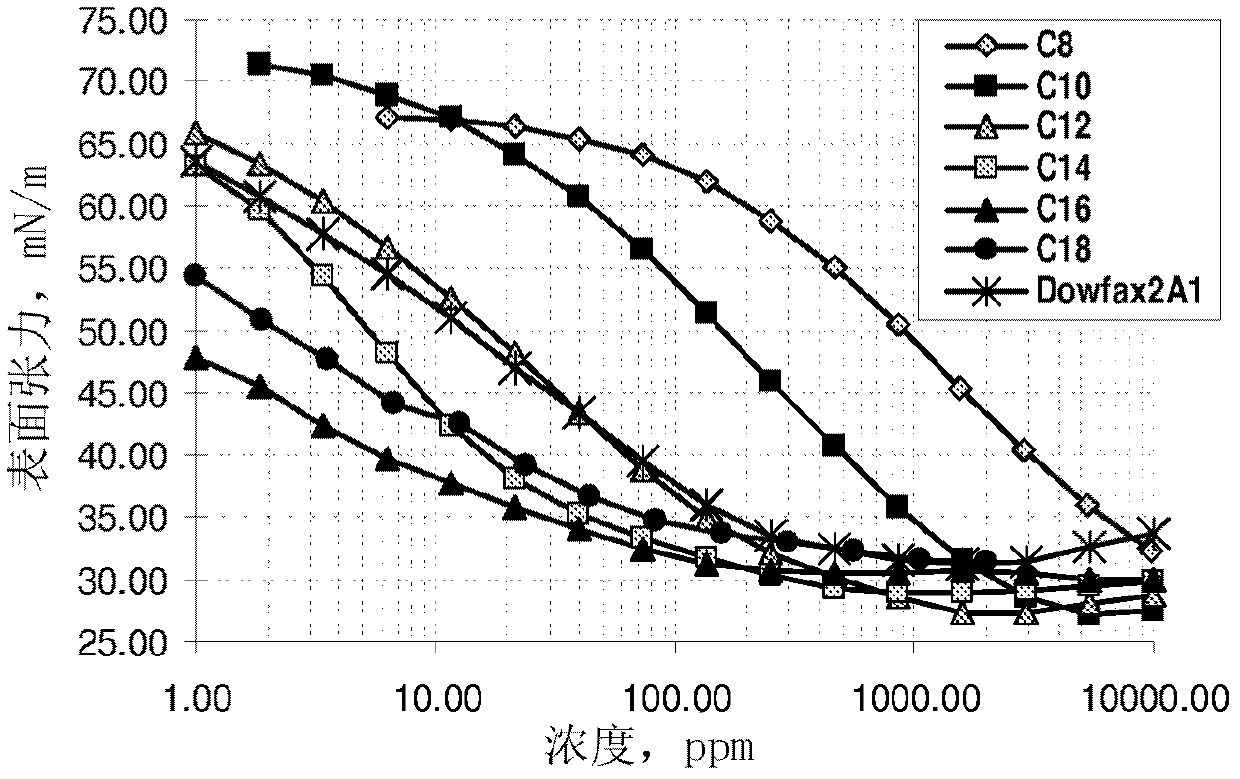
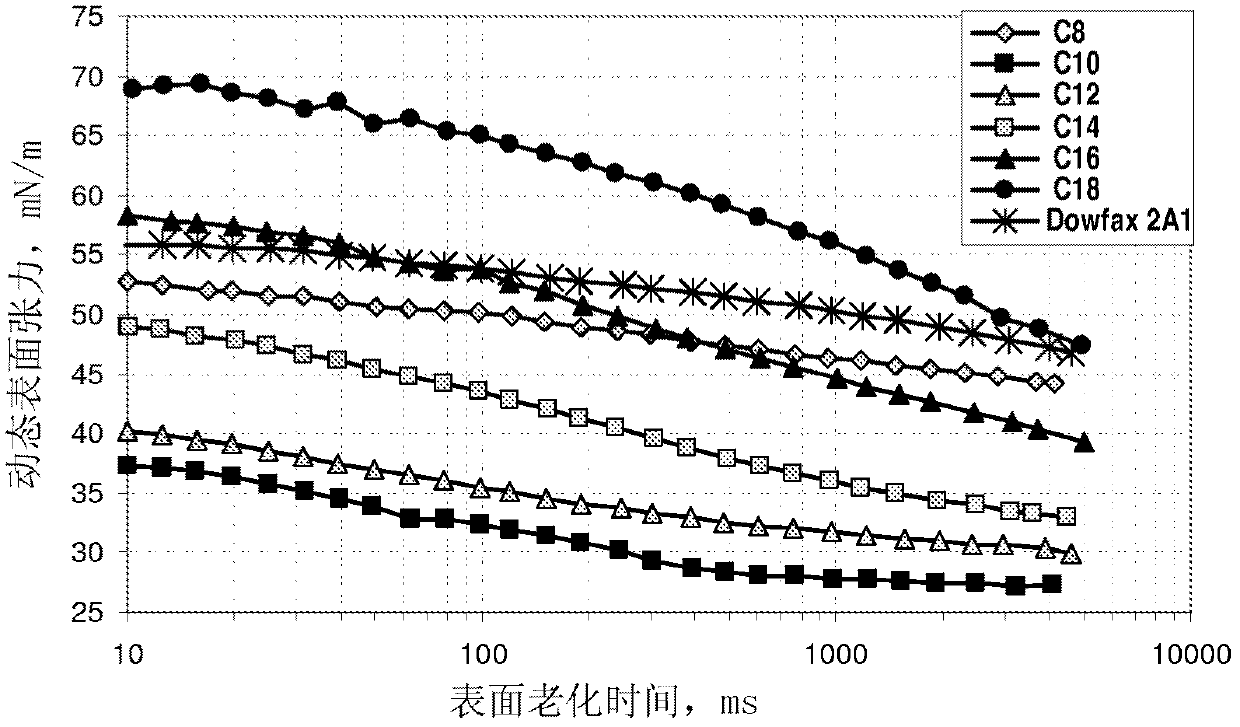
Sulfonate surfactants and methods of preparation and use
A technology of sodium disulfonate and preparations, applied in the directions of surface active detergent compositions, chemical instruments and methods, pharmaceutical formulations, etc., can solve problems such as lack of selection and performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Etherification of α-olefins with 1,3-dichloro-2-propanol
[0101] Exemplary ether compounds, precursors to compounds of formula I, can be prepared by the following schemes.
[0102] A Dean-Stark trap with a bottom drain of glass wool plug for retaining resin beads was charged with 16.2 g of DOWEX DR-2030 resin, which was charged with 11.5 g of 1,3- Wet with dichloro-2-propanol, and connect the apparatus to a 1-L round bottom flask. The flask was charged with 1.1 mol of alpha-olefin and 139.7 g of 1,3-dichloro-2-propanol (1.17 mol in total). Vacuum was applied and the 1-L flask was heated to concentrate the distillate from the 1-L flask into the Dean Stark trap containing the warmed resin and back to the 1-L flask. The temperature in the 1-L flask rose as distillation continued. The reaction mixture was purified by distillation to provide the alkyl 1,3-dichloropropyl ether.
Embodiment 2
[0104] Preparation of sulfonates from sodium sulfite / sodium metabisulfite
[0105] Exemplary surfactants of the present invention can be manufactured by the following schemes.
[0106] A 2 L Parr reactor was charged with 0.456 mol alkyl 1,3-dichloropropyl ether (from Example 1), 0.783 mol sodium sulfite, 0.180 mol sodium metabisulfite, 0.289 mol sodium carbonate and 590 g water. After nitrogen purge and pressure check, the system was heated to 200°C for 20 hours. The pressure after reaching temperature was 250 psig. The solution was cooled to ambient temperature and discharged to provide the reaction product.
Embodiment 3
[0108] Etherification of 1-dodecene with 1,3-dichloro-2-propanol
[0109]The ether, made essentially according to the protocol described in Example 1, was produced as follows. A 2-L reactive distillation apparatus was constructed as described here. A 2-L round bottom flask with a magnetic stirrer was fitted into a heating mantle and connected to a distillate receiver. Condensate the distillate into a side arm distillate receiver containing a magnetic stirrer and temperature probe. The valved line between the distillate receiver and the 2-L flask provides a nominal volume of approximately 100 mL in the distillate receiver. Liquid was pumped from the bottom of the distillate receiver to a 21 inch long and 3 / 4 inch diameter stainless steel tube fitted with a 90 μm screen filter on each end to provide a catalyst bed. A jacketed system covering the catalyst-containing tubes was heated using a recirculating hot oil bath. The outlet of the catalyst bed returns the liquid to the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
| greyscale | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com