Method for selectively recovering americium from an aqueous nitric phase
一种水相中、选择性的技术,应用在回收利用技术、化学仪器和方法、放射性净化等方向,能够解决情形复杂等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] Example 1: Detailed Discussion of a First Exemplary Embodiment of the Method of the Invention
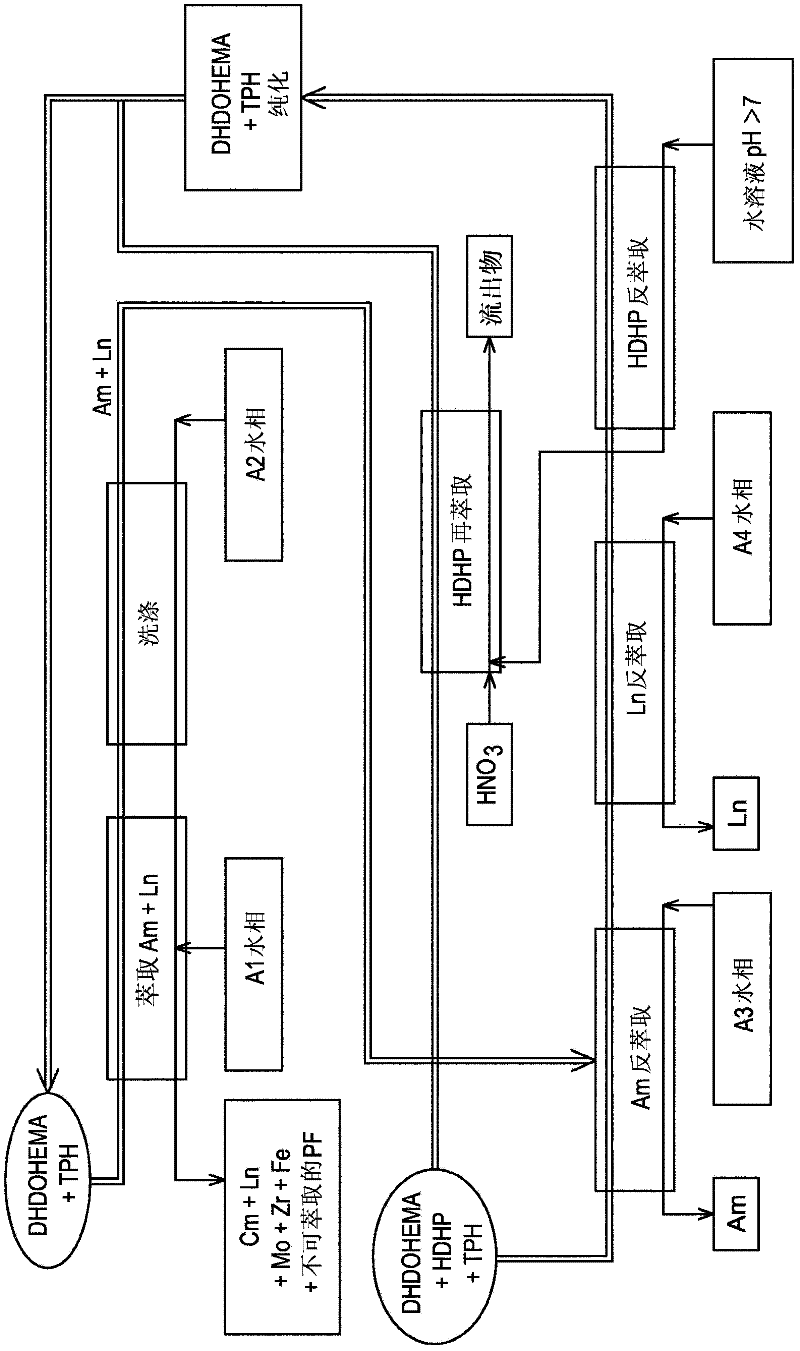
[0134] refer to figure 1 , which schematically illustrates a first exemplary embodiment of the invention, is designed to process on an industrial scale originating from PUREX or COEX TM The raffinate of the first purification cycle of the process, with the aim of selectively recovering the americium present in this raffinate.
[0135] The latter (the above raffinate), below and figure 1 Indicated by the A1 aqueous phase, is an aqueous solution with strong nitric acidity containing americium, curium, lanthanides (La, Ce, Pr, Nd, Sm, Eu, Gd, ...), different from lanthanides fission products (Mo, Zr, Ru, Rd, Pa, Y, ...) and other metal elements such as iron and chromium that are neither lanthanides nor fission products.
[0136] On the other hand, it does not contain uranium, plutonium and neptunium, or if any of these elements are present, it is only present in trace amounts...
Embodiment 2
[0168] Example 2: Detailed Discussion of a Second Exemplary Embodiment of the Method of the Invention
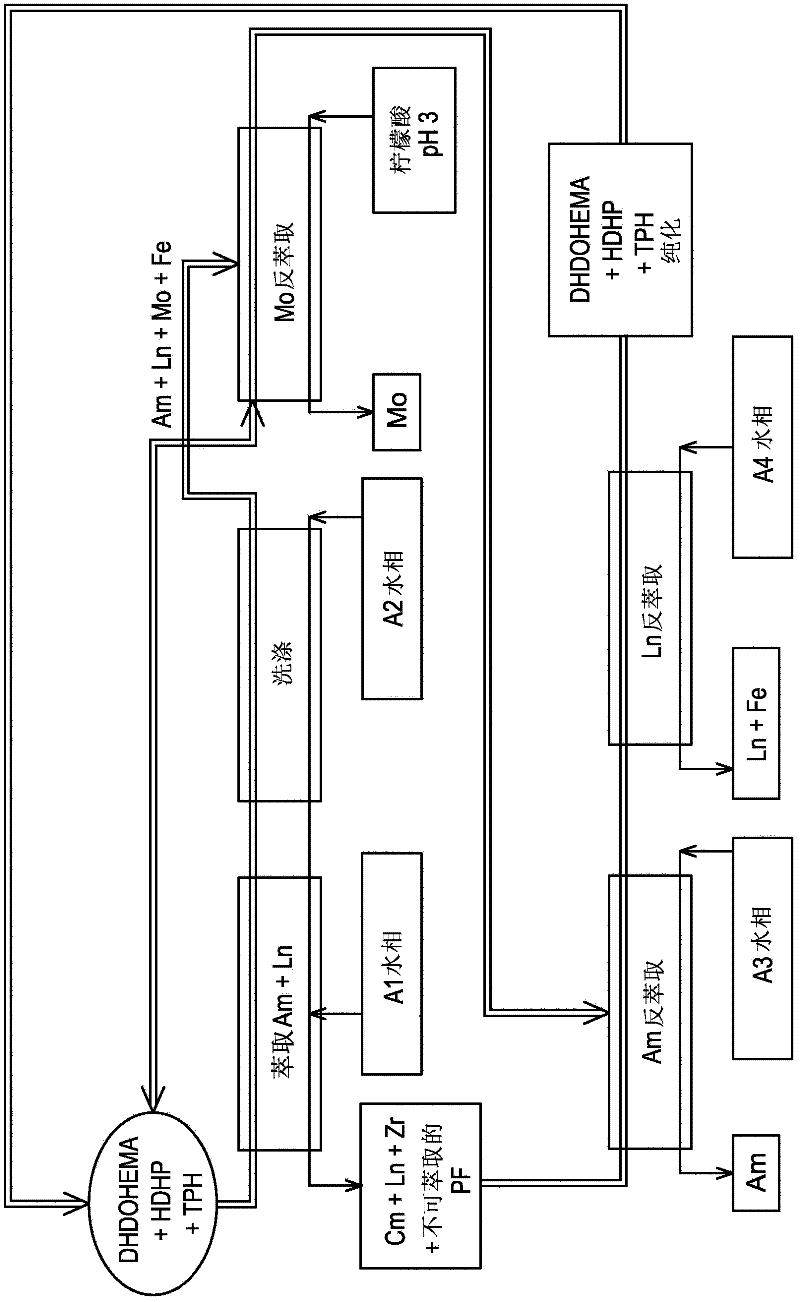
[0169] now refer to figure 2 , which schematically shows a second exemplary embodiment of the method of the present invention, which is also designed for processing on an industrial scale originating from PUREX or COEX TM The raffinate of the first purification cycle of the process, with the aim of selectively recovering the americium present in this raffinate, but wherein an acid extractant is present in the organic phase of all steps of the process.
[0170] Also, in this second embodiment, each cycle of the method includes the following six steps:
[0171] 1) extracting americium and a part of lanthanides present in the A1 aqueous phase by utilizing an organic phase containing both a solvating extractant and an acid extractant;
[0172] 2) washing the organic phase resulting from this extraction;
[0173] 3) selective stripping of molybdenum from the organic phase der...
Embodiment 3
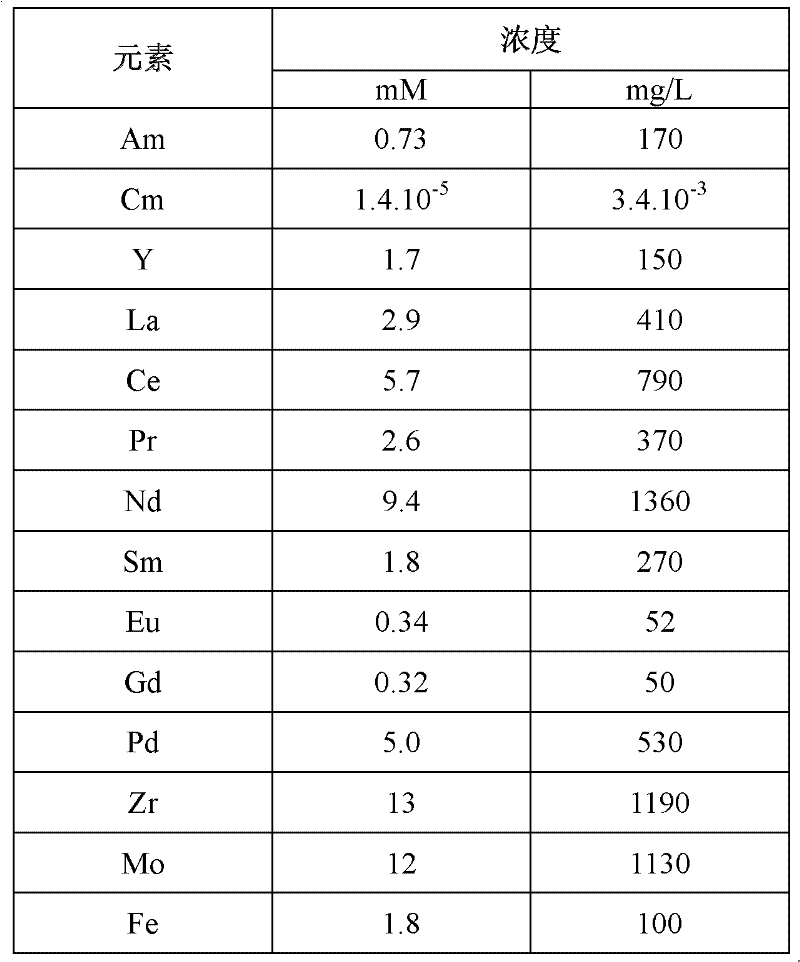
[0192] Embodiment 3: verification of embodiment 1 and embodiment 2
[0193] In the following, determine the partition coefficient of the metal element:
[0194] - in the case of americium and curium, by calculating the ratio of the activity of these elements in a given organic phase relative to the activity of these same elements in an aqueous phase which has been placed in contact with this organic phase;
[0195] - in the case of other metallic elements, by calculating the difference between the initial and final concentrations of these elements in a given aqueous phase, and by calculating the difference between this difference and the initial concentrations of these same elements in these same aqueous phases ratio between.
[0196] All measurements of the activity of americium and curium were carried out by alpha spectrometry, while all measurements of the concentrations of other metal elements were carried out by atomic emission spectroscopy using inductively coupled plas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com