Preparation method of histamine dihydrochloride
A technology of histamine dihydrochloride and hydrochloric acid, which is applied in the direction of medical preparations, drug combinations, and pharmaceutical formulas containing active ingredients, and can solve the problem that the product quality cannot meet the pharmaceutical level, cannot be used as a pharmaceutical preparation raw material, and is not suitable for Industrial production and other issues, to achieve the effect of facilitating large-scale industrial production, easy recycling, and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
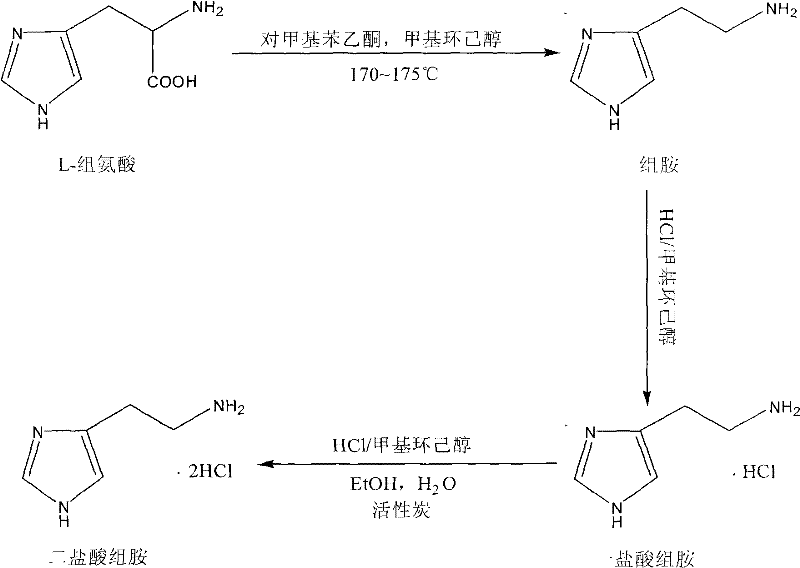
[0023] Add 150g of L-histidine, 40ml of p-methylacetophenone and 1200ml of trans-3-methylcyclohexanol into a 2000ml three-necked flask equipped with mechanical stirring, thermometer and reflux device, replace the air with vacuum and nitrogen, and pass through the whole process. Protected under nitrogen, protected from light, heated to 170-175°C and refluxed for 24 hours. Then slowly lower the temperature to below 30°C, add dropwise a methylcyclohexanol solution containing 21.2g of hydrochloric acid, and control the temperature below 45°C. Methylcyclohexanol containing hydrochloric acid can be prepared by passing HCl gas into 180g of trans-3-methylcyclohexanol at 20-25°C, and the mass percentage of hydrochloric acid is 20-25%. After the dropwise addition is complete, stir at 20-25°C for 4 hours. Filter, collect mother liquor, filter cake rinse 3 times with 100ml dichloromethane, collect dichloromethane eluate. The filter cake was dried under vacuum for 4 hours to obtain an of...
Embodiment 2
[0025] Add 150g of L-histidine, 40ml of p-methylacetophenone and 1000ml of cis-3-methylcyclohexanol to a 2000ml three-necked flask equipped with mechanical stirring, a thermometer and a reflux device, and replace the air with vacuum and nitrogen. Protected under nitrogen, protected from light, heated to 170-175°C and refluxed for 24 hours. Slowly lower the temperature to below 30°C, add dropwise a 3-methylcyclohexanol solution containing 21.2g of hydrochloric acid, and control the temperature below 45°C. 3-methylcyclohexanol containing hydrochloric acid can be prepared by passing HCl gas into 180 g of cis-3-methylcyclohexanol at 20-25° C., and the mass percentage of hydrochloric acid is 20-25%. After the dropwise addition is complete, stir at 20-25°C for 4 hours. Filter to collect the mother liquor, rinse the filter cake 3 times with 100ml petroleum ether, and collect the petroleum ether eluent. The filter cake was dried under vacuum for 4 hours to obtain an off-white solid....
Embodiment 3
[0027]Add 150gL-histidine, 40ml p-methylacetophenone and 900ml 4-methylcyclohexanol (mixture of cis and trans isomers) in the 2000ml there-necked flask equipped with mechanical stirring, thermometer and reflux device, use vacuum and Replace the air with nitrogen, protect the whole process with nitrogen, avoid light, and heat to 170-175°C for reflux reaction for 24h. Slowly lower the temperature to below 30°C, add dropwise a 4-methylcyclohexanol solution containing 21.2g of hydrochloric acid, and control the temperature below 45°C. 4-methylcyclohexanol containing hydrochloric acid can be prepared by passing HCl gas into 180g of 4-methylcyclohexanol (mixture of cis and trans isomers) at 20-25°C, and the mass percentage of hydrochloric acid is 20 ~25%. After the dropwise addition is complete, stir at 20-25°C for 4 hours. Filter, collect mother liquor, filter cake rinse 3 times with 100ml n-hexane, collect n-hexane eluate. The filter cake was dried under vacuum for 4 hours to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com