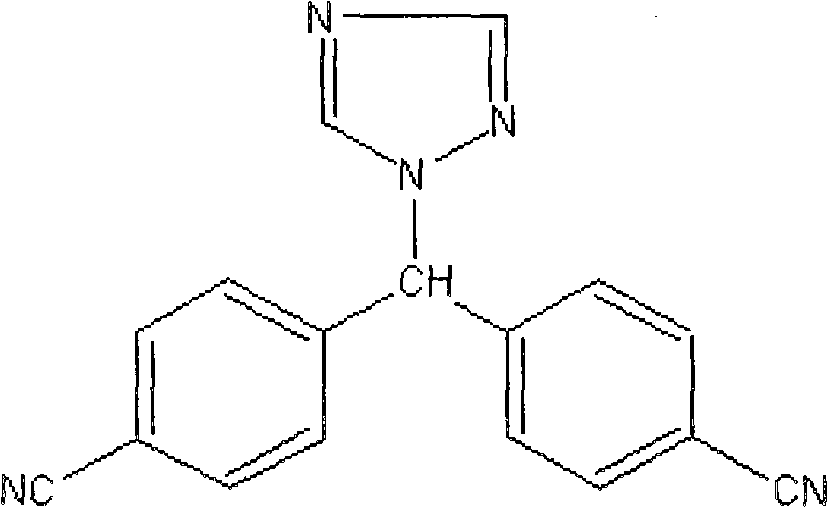
Letrozole tablet with high dissolubility and preparation method thereof
A technology for letrozole tablets and dissolution rate is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, medical preparations containing active ingredients, etc. Achieve the effect of improving bioavailability, saving energy and increasing absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
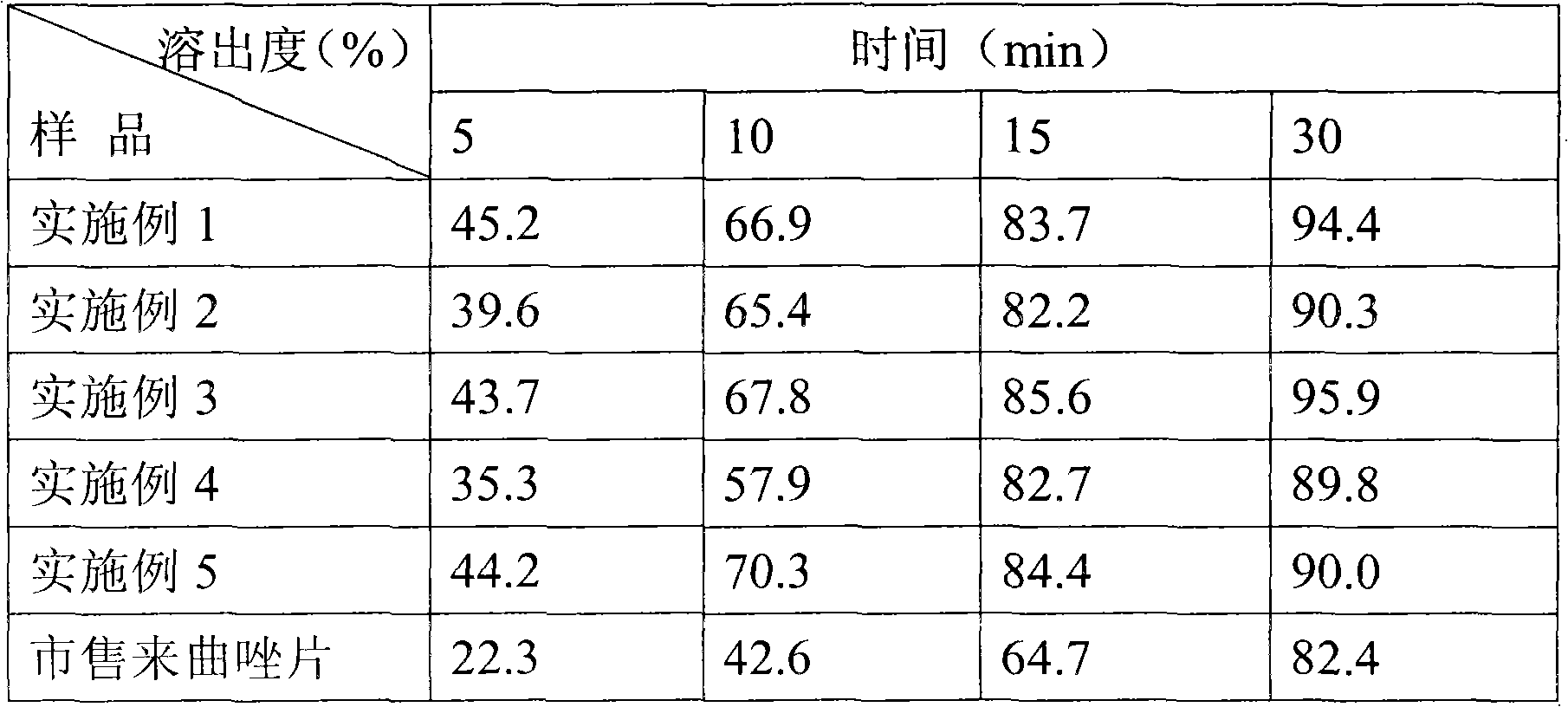
[0033] [Example 1] Prescription composition and preparation of letrozole tablets with high dissolution rate
[0034] Prescription A total of 8000 tablets
[0035] Letrozole 20g
[0036] Polyvinylpyrrolidone K30 35g
[0037] Tween 80 15g
[0038] Mannitol 30g
[0039] Lactose 500g
[0040] Microcrystalline Cellulose 450g
[0041] Cross-linked polyvinylpolypyrrolidone 40g
[0043] Preparation method: Add the prescribed amount of polyvinylpyrrolidone K30 (PVP K30), Tween 80, and mannitol into 400ml of water, stir to form a uniform solution, add letrozole into the solution, and disperse it uniformly for 5 minutes with ultrasonic to obtain a suspension ; Lactose, microcrystalline cellulose, and cross-linked polyvinylpolypyrrolidone (PVPP) of the above-mentioned prescription quantity are mixed uniformly, and the above-mentioned suspension is sprayed into the mixed auxiliary material by a spray gun with a peristaltic pump, and stirred while spray...
Embodiment 2
[0044] [Example 2] Prescription Composition and Preparation of High Dissolution Letrozole Tablets
[0045] Prescription A total of 8000 tablets
[0046] Letrozole 20g
[0047] Polyvinylpyrrolidone K30 20g
[0048] Tween 80 20g
[0049] Mannitol 20g
[0050] Lactose 600g
[0051] Microcrystalline Cellulose 350g
[0052] Cross-linked polyvinylpolypyrrolidone 30g
[0054] Preparation method: Add the prescribed amount of polyvinylpyrrolidone K30 (PVP K30), Tween 80, and mannitol into 400ml of water, stir to form a uniform solution, add letrozole to the solution, and disperse it evenly for 10 minutes by ultrasonication to obtain a suspension ; Lactose, microcrystalline cellulose, and cross-linked polyvinylpolypyrrolidone (PVPP) of the above-mentioned prescription quantity are mixed uniformly, and the above-mentioned suspension is sprayed into the mixed auxiliary material by a spray gun with a peristaltic pump, and stirred while spraying, to mak...
Embodiment 3
[0055] [Example 3] Prescription Composition and Preparation of High Dissolution Letrozole Tablets
[0056] Prescription A total of 8000 tablets
[0057] Letrozole 20g
[0058] Polyvinylpyrrolidone K30 25g
[0059] Tween 80 20g
[0060] Mannitol 25g
[0061] Lactose 600g
[0062] Microcrystalline Cellulose 350g
[0063] Cross-linked polyvinylpolypyrrolidone 25g
[0065]Preparation method: Add the prescribed amount of polyvinylpyrrolidone K30 (PVP K30), Tween 80, and mannitol into 500ml of water, stir to form a uniform solution, add letrozole into the solution, and disperse it evenly for 10 minutes with ultrasonic to obtain a suspension ; Lactose, microcrystalline cellulose, and cross-linked polyvinylpolypyrrolidone (PVPP) of the above-mentioned prescription quantity are mixed uniformly, and the above-mentioned suspension is sprayed into the mixed auxiliary material by a spray gun with a peristaltic pump, and stirred while spraying, to make ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com