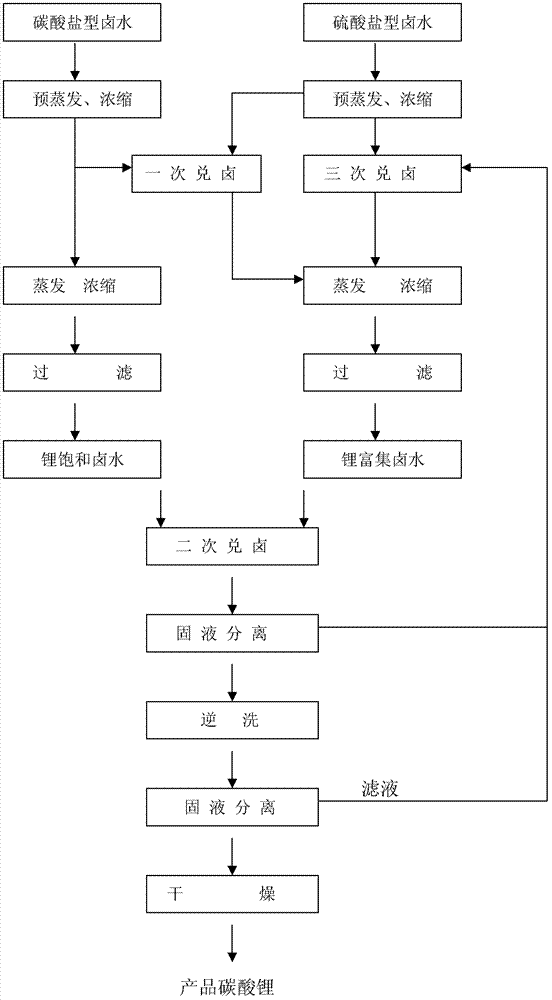
Producing method for preparing lithium carbonate by taking carbonate type brine and sulphate type brine as raw material and by repeatedly mixing brine
A carbonate-type and sulfate-type technology, applied in the direction of lithium carbonate;/acid carbonate, magnesium carbonate, etc., can solve the problems of high solubility of lithium carbonate, production difficulties, and low solubility of lithium carbonate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
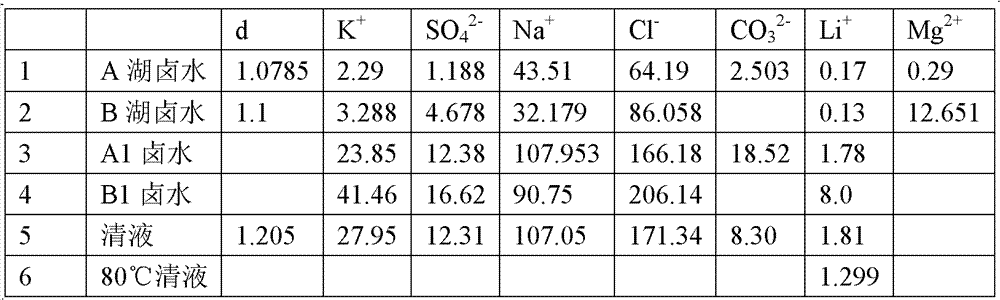
[0071] 1. Take the brine from Lake A in the Ngari area of Tibet, the density d=1.0785, and the composition is as follows (g / l):
[0072] K + Mg 2+ Na + Li + SO 4 2- CO 3 2- Cl -
[0073] 2.29 0.29 43.51 0.17 1.188 2.50 364.19
[0074] When the brine is evaporated to a lithium ion content of 1.78 g / l, its composition is as follows:
[0075] K + Na + Li + SO 4 2- CO 3 2- Cl -
[0076] 23.85 107.953 1.78 12.375 18.52 166.177 (g / l)
[0077] Get this bittern 50L, set aside. The halogen is called A 1 .
[0078] 2. Take brine from Lake B in Ngari, Tibet, with density d=1.1, and the composition is as follows (g / l):
[0079] K + Mg 2+ Na + Li + SO 4 2- Cl -
[0080] 3.288 12.651 32.179 0.13 4.678 86.058
[0081] It is mixed with the brine of Lake A in a ratio of A: B = 94.6: 5.4 to be brine (the calculation basis for the ratio of brine is:
[0082] Magnesium ion: carbonate grou...
Embodiment 2
[0095] take A 1 7L brine, take B 1 Mix 2L of brine, stir and heat to 80°C, stir for a period of time, and filter while hot to obtain a clear liquid. The lithium ion content of the serum was 1.299g / l. After simple material balance calculation, B 1 The singlet precipitation rate of lithium brine is 83.76%, A 1 The single-line precipitation rate of lithium brine is 27.02%. The lithium carbonate precipitated in this example is a crude product, which can be obtained by backwashing and drying.
[0096] Note: The total acquisition rate of lithium: the leakage of brine in the salt pan caused by natural evaporation and the entrainment of other salts in the salt pan evaporation are unavoidable in any salt field production, and usually do not affect the calculation of the total production rate of the workshop. If According to the above routine, when calculating the total lithium acquisition rate in this process, except for running, escaping, dripping, and leaking in the production wo...
Embodiment 3
[0098] 1. Take the brine from Lake C in Ngari, Tibet, with a density of d=1.13, and the composition is as follows (g / l):
[0099] K + Mg 2+ Ca 2+ Na + Li + SO 4 2- CO 3 2- Cl -
[0100] 16.122 0.004 0.002 60.728 0.13 16.700 5.559 89.572
[0101] When the brine is evaporated to a lithium ion content of 1.78 g / l, its composition is as follows:
[0102] K + Na + Li + SO 4 2- CO 3 2- Cl -
[0103] 49.04 107.12 1.78 15.89 75.97 197.945 (g / l)
[0104] Take 5L of the brine and set aside. The halogen is called C 1 .
[0105] 2. Take the brine from Lake D in Ngari, Tibet, with a density of d=1.1275, and the composition is as follows (g / l):
[0106] K + Mg 2+ Ca 2+ Na + Li + SO 4 2- CO 3 2- Cl - HCO 3 -
[0107] 10.894 3.64 0.108 57.429 0.16 4.480 0.790 105.615 0.18
[0108] Mix with the brine of Lake A in the ratio C: D=60.1:39.9 and mix it with brine (the calcu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com