Method for synthetizing indanone compound
A synthesis method and technology of ketone compounds, which are applied in the field of preparation of organic compounds, can solve the problems of serious pollution and difficult operation, and achieve the effects of simple reaction operation, less environmental pollution, industrialization and sustainable development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
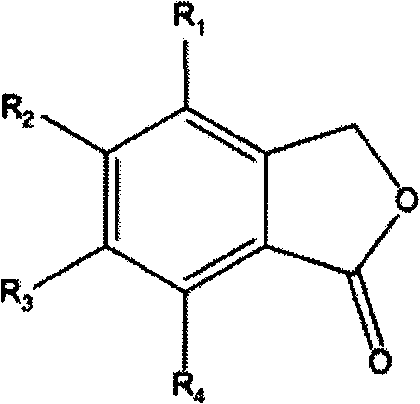
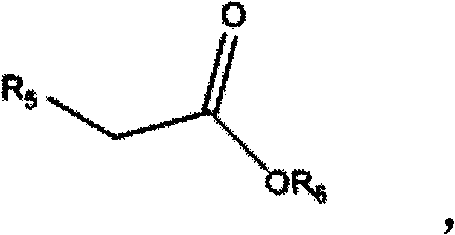
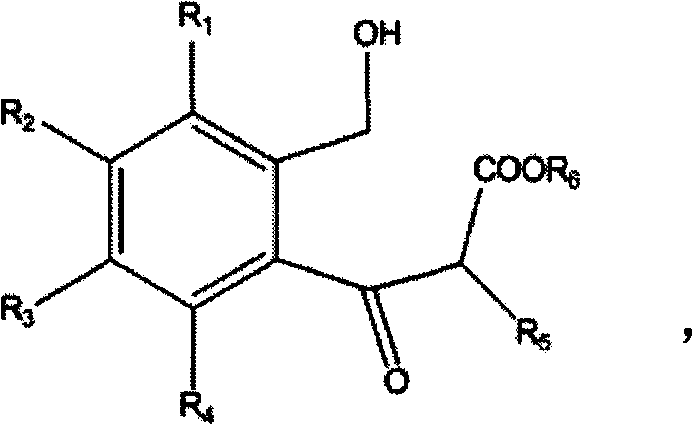
[0039] Add 600ml DMSO and 1mol sodium hydride into a 2L reaction flask, add dropwise a mixture of 1mol ethyl propionate and 1mol isobenzofuran-1(3H)-one under mechanical stirring, keep the reaction temperature at -10-0°C, drop After the addition was completed, the temperature was slowly raised to room temperature and the reaction was continued for 12 hours. After the reaction was completed, 800 ml of water was added to dilute, then extracted with n-hexane, and the solvent was distilled off to obtain 184.1 g of 1,3-dicarbonyl compound.
[0040] Dissolve the above-mentioned 184.1g dicarbonyl compound with 600ml ethylene dichloride, then add 2% mole catalyst iodine, and react with mechanical stirring for 14 hours. After the layer was washed once with water, the solvent was distilled off to obtain 111.6 g of 2-methyl-2,3-dihydro-1-indanone with a yield of 76.3%. The spectrogram data is as follows: 1 H NMR (500MHz, CDCl 3 ): δ=1.30(d, 3H), δ=2.54-2.76(d, 2H), δ=3.34-3.46(m, 1H), ...
Embodiment 2
[0042] Replace the isobenzofuran-1(3H)-one in Example 1 with an equimolar amount of 4,7-dimethyl-3-phenylisobenzofuran-1(3H)-one, and the other conditions are the same In Example 1, 196.3 g of 2,4,7-trimethyl-3-phenyl-2,3-dihydro-1-indanone was obtained, with a yield of 78.4%. The spectrogram data is as follows: 1 H NMR (300MHz, CDCl 3 ): δ=1.23(d, 3H), δ=2.34(s, 6H), δ=2.58-2.83(dd, 2H), δ=3.55(dd, 1H), δ=7.41-7.58(m, 5H) , δ=7.80(s, 1H).
Embodiment 3
[0044] The isobenzofuran-1(3H)-one in Example 1 is replaced with 5,7-dimethylisobenzofuran-1(3H)-one in an equimolar amount, and other reaction conditions are the same as in Example 1, 138.5 g of 2,5,7-trimethyl-2,3-dihydro-1-indanone was obtained with a yield of 79.5%. The spectrogram data is as follows: 1 H NMR (400MHz, CDCl 3 ): δ=1.27(d, 3H), δ=2.36(s, 3H), δ=2.58(s, 3H), δ=3.25(dd, 1H), δ=6.90(s, 1H), δ=7.04 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com