Stable sodium hypochlorite solution and preparation method thereof
A technology of sodium hypochlorite solution and stability, applied in the directions of hypochlorous acid, hypochlorite, etc., can solve problems such as poor stability of sodium hypochlorite solution, and achieve the effects of no potential toxicity problem, convenient use and stable concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
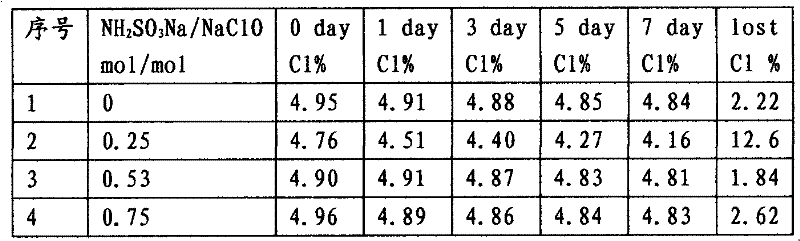
[0017] Amino compounds such as sulfamic acid, urea and glycine were used as alternative stabilizers for sodium hypochlorite solution. Dissolve 9.7g (0.10mol) of sulfamic acid, 6.0g (0.10mol) of urea and 7.7g (0.10mol) of aminoacetic acid in 50ml of deionized water, adjust the pH to 13.5 with 2.0mol / L sodium hydroxide solution, Dilute to 120g with deionized water, add 78.2g (0.10mol) of sodium hypochlorite solution with an available chlorine content of 10% respectively, and measure the available chlorine content in the mixed solution after mixing as shown in Table 1.
[0018] Table 1 The impact of amino compound types on available chlorine in sodium hypochlorite solution
[0019] serial number
[0020] In the above experiments, before and after the mixing of sodium sulfamate solution and sodium hypochlorite solution, the total amount of available chlorine in the solution is almost unchanged, and no obvious chemical reaction phenomenon occurs after mixing, and the char...
Embodiment 2
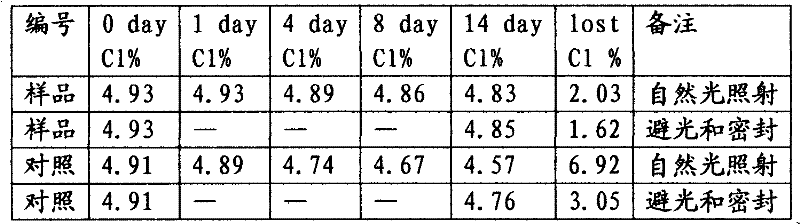
[0022] Get 71.4g, 147g and 210g of 30% sodium sulfamate solution respectively, add 80g of 30% sodium tripolyphosphate solution respectively, then add 274g (0.70mol) of industrial sodium hypochlorite solution with 10% available chlorine content, and use 50% sodium hydroxide Adjust the alkalinity of the solution, stir to obtain 5.0% available chlorine content, 5.0% sodium tripolyphosphate content, 2.0% free alkali content, and sodium hypochlorite solution sample liquid containing 0.18mol, 0.37mol, and 0.53mol of sodium sulfamate stabilizer. Store it at room temperature of 25°C, regularly take samples to measure the available chlorine content in the solution and calculate the cumulative decline rate of available chlorine in Table 2.
[0023] The influence of table 2 stabilizer addition on the stability of sodium hypochlorite solution
[0024]
[0025] In the above experiments, when the mol ratio of sodium sulfamate / sodium hypochlorite was 0.25, the available chlorine in the so...
Embodiment 3
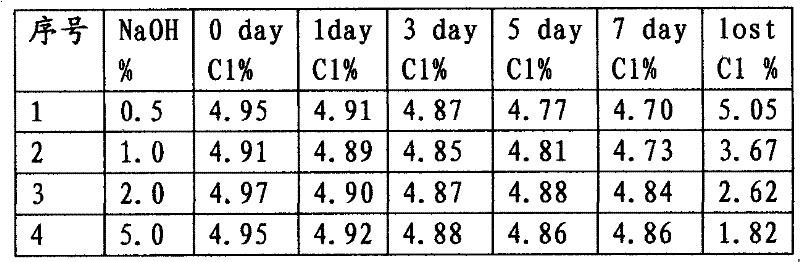
[0027] In 147g (0.37mol) of 30% sodium sulfamate solution, add 80g of 30% sodium tripolyphosphate solution, then add 250g (0.70mol) of industrial sodium hypochlorite solution with 10% available chlorine content, adjust the solution with 50% sodium hydroxide Alkalinity, stir to obtain available chlorine content 5.0%, sodium tripolyphosphate content 5.0%, free alkali content is respectively 0.5%, 1.0%, 2.0% and 5.0% sodium hypochlorite solution sample solution 500g. Place it at room temperature of 25°C, periodically take samples to measure the available chlorine content of the solution and calculate the cumulative decline rate of available chlorine, as shown in Table 3.
[0028] The influence of table 3 free alkali content on the stability of sodium hypochlorite solution
[0029]
[0030]As seen from the above results, when the solution free alkali content was 0.5%, the stability of the sodium hypochlorite solution was poor; when the solution free alkali content was greater t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com