Method for producing 2-alkylene grease cyclic ketone by adopting bionic catalytic system
An alkylene ester, biomimetic catalysis technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of catalysts that cannot be recycled and reused, loss of raw materials and products, waste, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
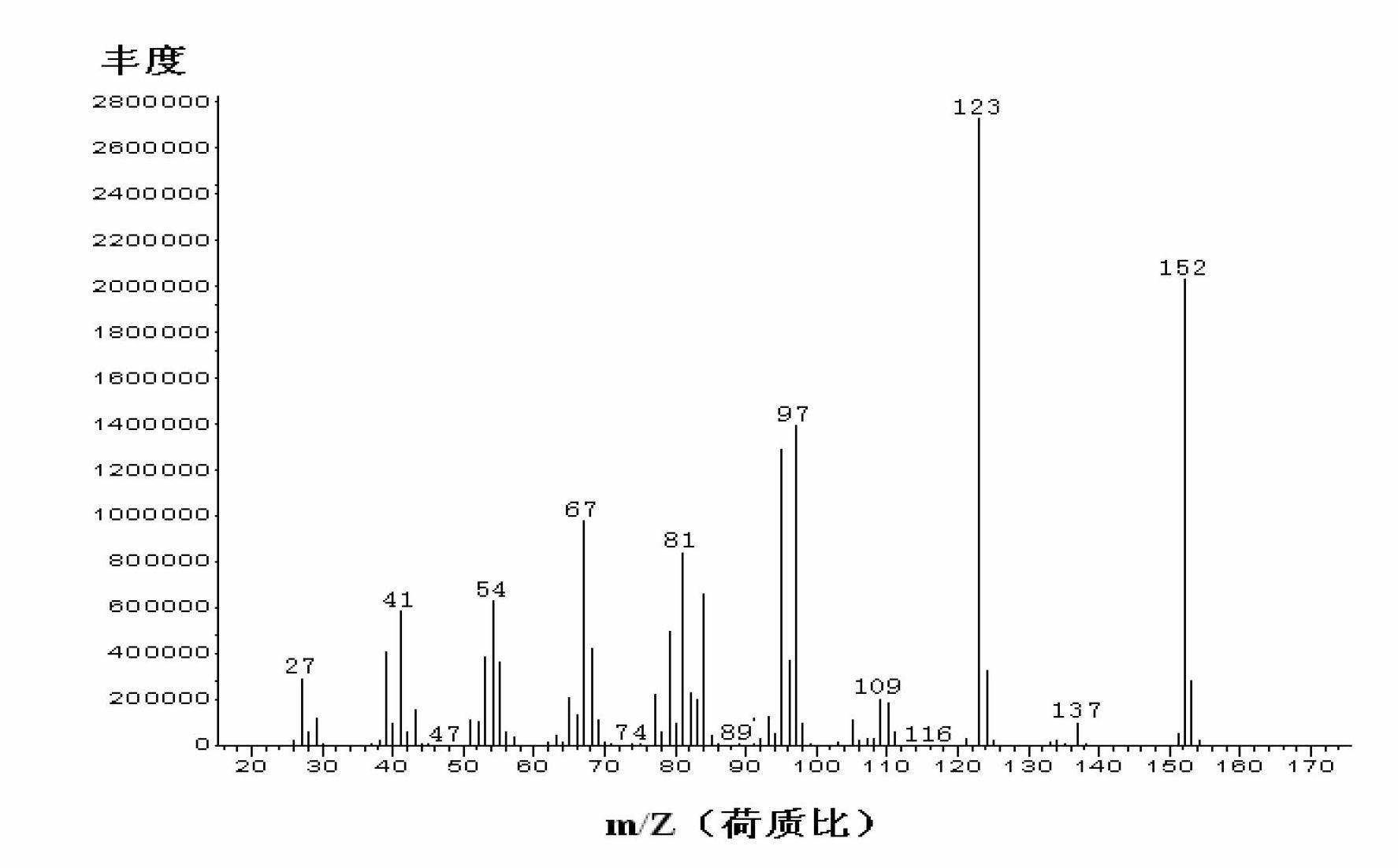
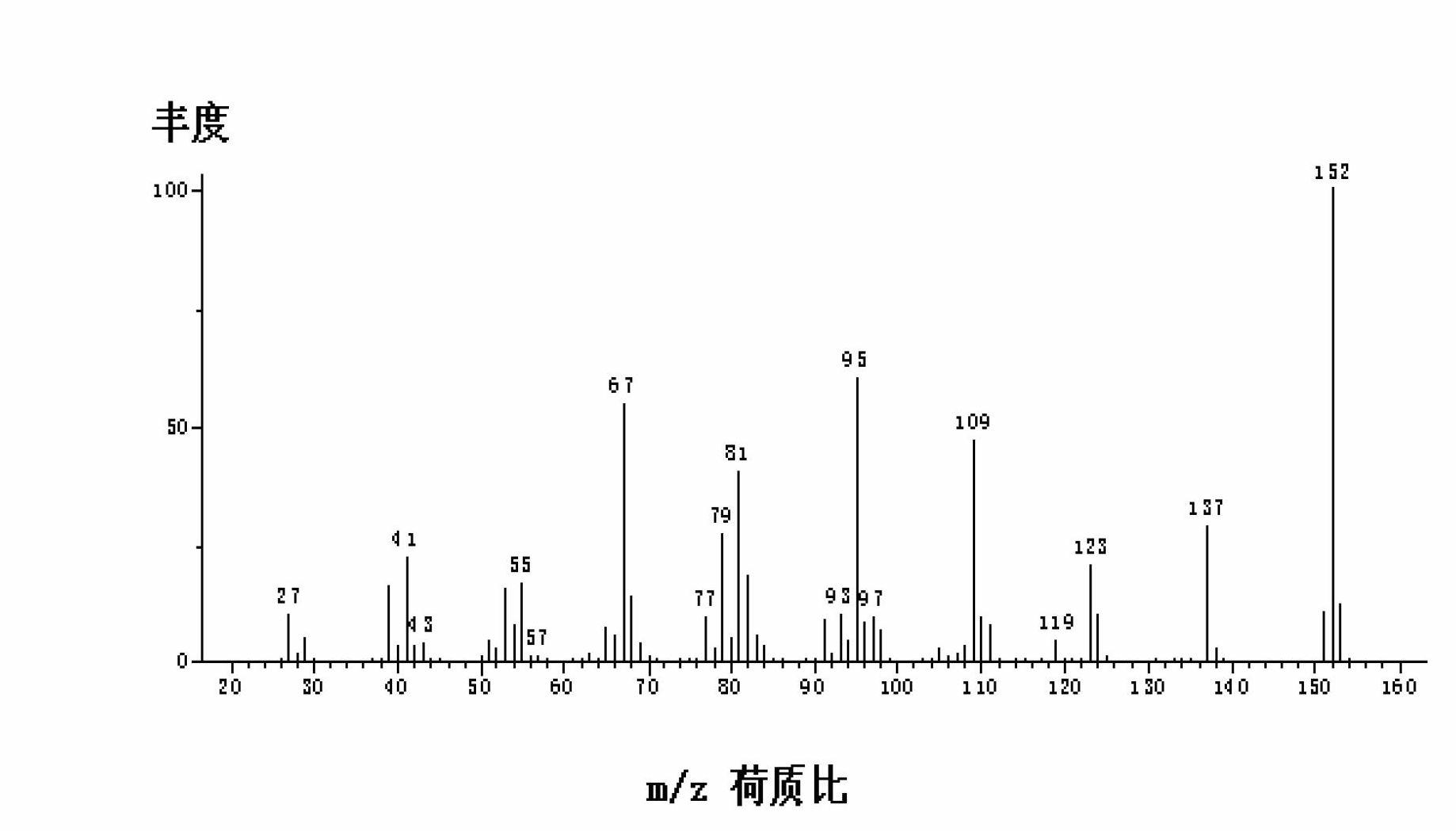
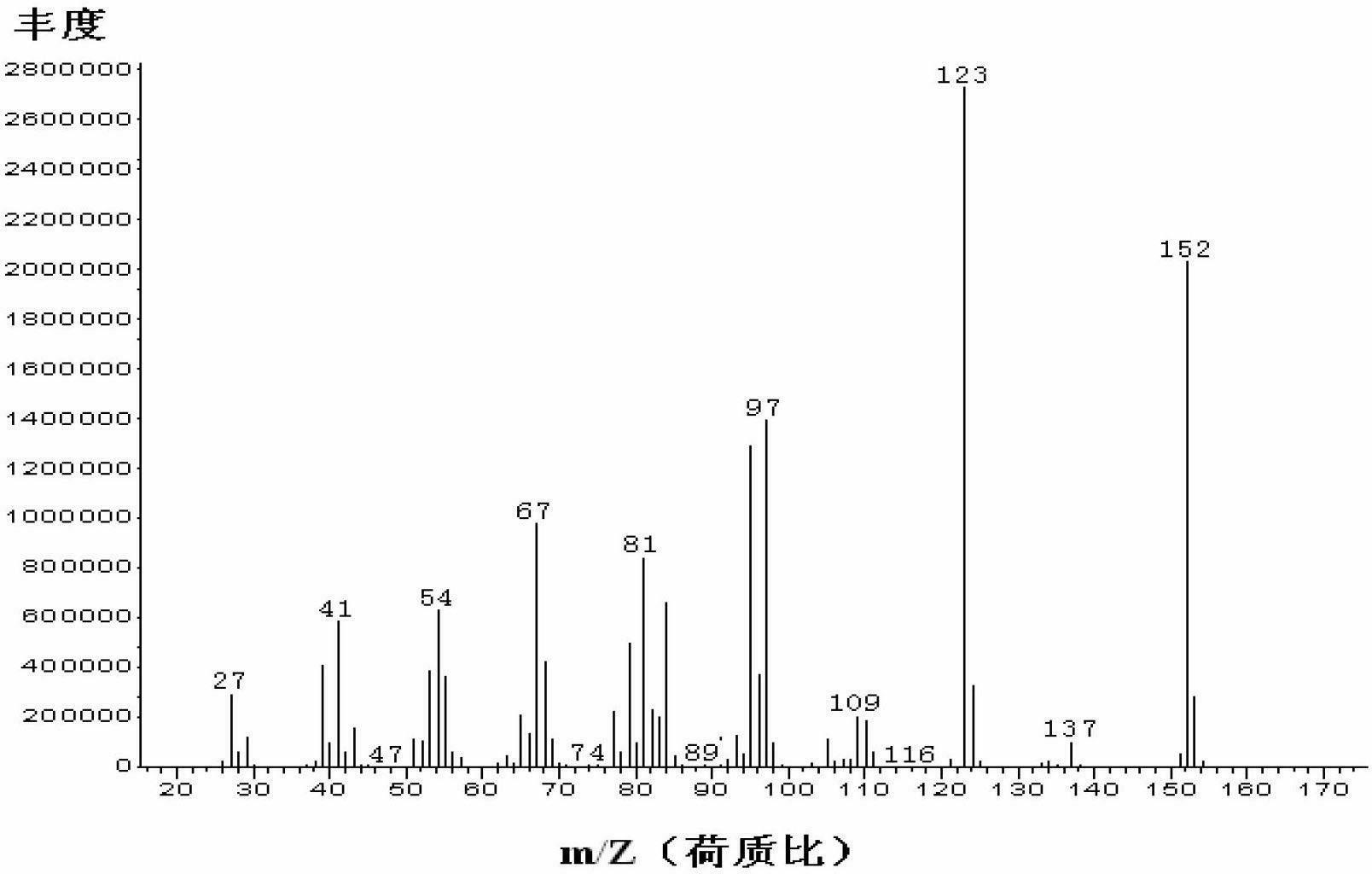
Image
Examples
Embodiment 1
[0051] Example 1 A method for synthesizing 2-alkylene cycloaliphatic ketones (such as using one of cyclobutanone, cyclopentanone or cyclohexanone, two of them, or a mixture of the three) using clean technology comprises the following steps:
[0052] (1) Put a certain amount of alicyclic ketones, fatty aldehydes, amine-containing substances, protonic acids and metal ion-containing aqueous solutions into the reactor at one time and mix them at room temperature, adjust the pH to 3~4, and heat up to 50°C under light. Continue to react at this temperature until the basic reaction of aliphatic aldehyde is complete;
[0053] (2) separating the catalyst system and organic phase containing amine-based substances from the reaction mixture obtained in step (1), and the catalyst system can be recycled;
[0054] (3) The organic phase that step (2) obtains adopts vacuum distillation to remove the light fraction containing unreacted cycloaliphatic ketone and some low boiling point substan...
Embodiment 2
[0056] Example 2 A method for synthesizing 2-alkylene cycloaliphatic ketones (such as using one of cyclobutanone, cyclopentanone or cyclohexanone, two of them, or a mixture of the three) using clean technology comprises the following steps:
[0057] (1) Put the alicyclic ketone, amino group-containing substances, protonic acid and metal ion-containing aqueous solution into the reactor at one time, adjust the pH to 5~6, heat up to 60°C under light, and add fat dropwise under stirring Aldehyde, then maintain at this temperature to continue the reaction until the basic reaction of fatty aldehyde is complete;
[0058] (2) separating the catalyst system and the organic phase containing the amine-based substance from the reaction mixture obtained in step (1), and the catalyst system can be recycled;
[0059] (3) The organic phase that step (2) obtains adopts vacuum distillation to remove the light fraction containing unreacted cycloaliphatic ketones and low boilers, and the light...
Embodiment 3
[0061] Example 3 A method for synthesizing 2-alkylene cycloaliphatic ketones (such as using one of cyclobutanone, cyclopentanone or cyclohexanone, two of them, or a mixture of the three) using clean technology comprises the following steps:
[0062] (1) First put a certain amount of alicyclic ketones and aliphatic aldehydes into the reactor, adjust the pH to 6~7, put in the mixture of amine-containing substances, protonic acids and metal ion-containing aqueous solutions under light and 30°C, and then maintain this Continue to react at a temperature until the basic reaction of the aliphatic aldehyde is complete;
[0063] (2) separating the catalyst system and the organic phase containing the amine-based substance from the reaction mixture obtained in step (1), and the catalyst system can be recycled;
[0064] (3) The organic phase that step (2) obtains adopts vacuum distillation to remove the light fraction containing unreacted cycloaliphatic ketones and low boilers, and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com