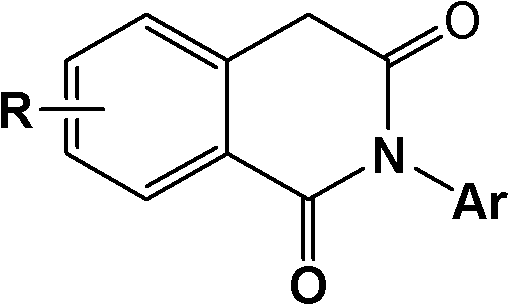
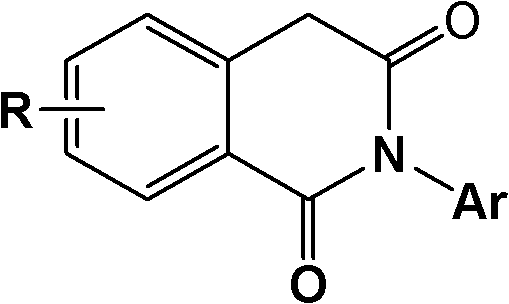
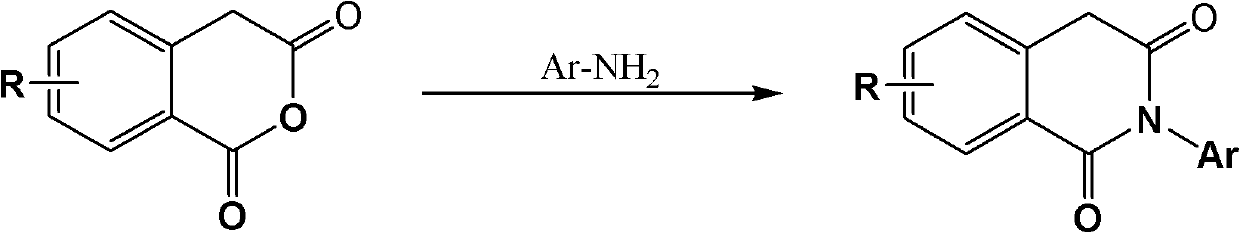2-aryl-1,3-isoquinoline diketone anti-tumor compound and synthesis method and application thereof
A technology of isoquinolinedione and its synthesis method, which is applied in the field of 2-aryl-1, can solve the problems of drug resistance and low clinical effective rate, and achieve the goal of easy synthesis, novel structure and inhibition of tumor cell proliferation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of 6,8-dimethoxy-2-phenyl-1,3-isoquinolinedione (1):
[0037] 3,5-dimethoxy homophthalic anhydride (prepared by reference J.Org.Chem.2003,68,5967-5973) 2.42g (10.89mmol), aniline 1.01g (10.89mmol) and glacial acetic acid ( 20 mL) into a 100 mL round-bottomed flask, the mixture was stirred and refluxed for 5 h under nitrogen protection, cooled to room temperature, slowly poured into water, left to stand overnight, and filtered with suction. The crude product was recrystallized from methanol to obtain 1.70 g of the product. Yield 52.6%. mp: 190-192°C.
Embodiment 2
[0039] Preparation of 6,8-onemethoxy-2-(4-methylphenyl)-1,3-isoquinolinedione (2):
[0040] With embodiment 1, difference is: replace aniline with 4-methylaniline. Yield 64.8%. mp: 160-163°C.
Embodiment 3
[0042] Preparation of 6,8-dimethoxy-2-(4-methoxyphenyl)-1,3-isoquinolinedione (3):
[0043] With embodiment 1, difference is: replace aniline with 4-methoxyaniline. Yield 84.8%. mp: 211-213°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com