Method for synthesizing dihydroxyl-terminated polysiloxane
The technology of a dihydroxyalkyl polysiloxane and a synthesis method is applied in the field of synthesis of double-terminal dihydroxyalkyl polysiloxanes, which can solve problems such as high cost, unfavorable application fields, and decreased yield of final products, and achieve improved Production efficiency, short total reaction time, and production cost reduction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
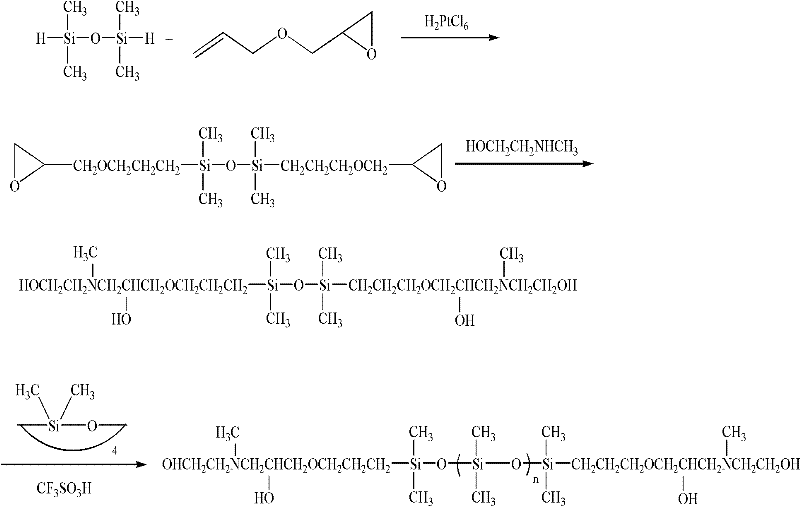
[0023] Hydrosilylation reaction (molar ratio of allyl glycidyl ether: tetramethyldisiloxane: chloroplatinic acid = 2.4: 1: 3.1 × 10 -4 ) In a 100ml four-necked bottle, add allyl glycidyl ether 27.38g, 10mL toluene and 40μL chloroplatinic acid 3.1×10 -5 mol of isopropanol solution, after passing nitrogen gas for 20 minutes, the temperature of the reaction system was raised to 100° C., and 13.41 g of tetramethyldisiloxane was added dropwise at this temperature, and the reaction was terminated after 8 hours of reaction. Vacuum distillation collected at 198-202°C (0.67KPa) to obtain 32.44g of 1,3-diepoxy-terminated disiloxane with a yield of 89.6%.
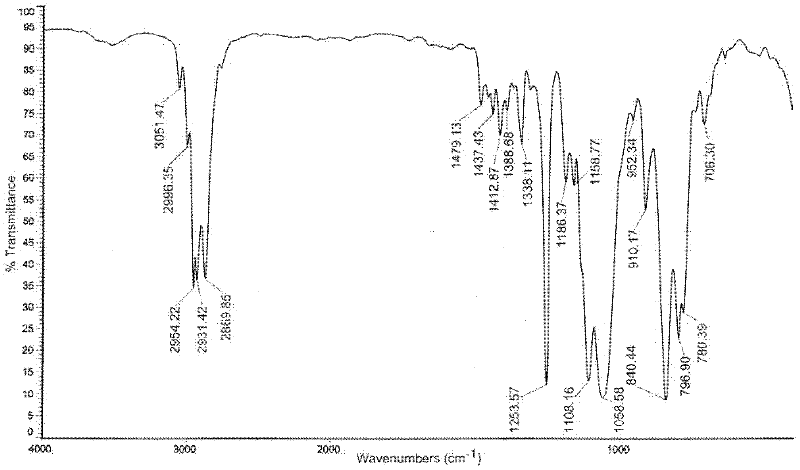
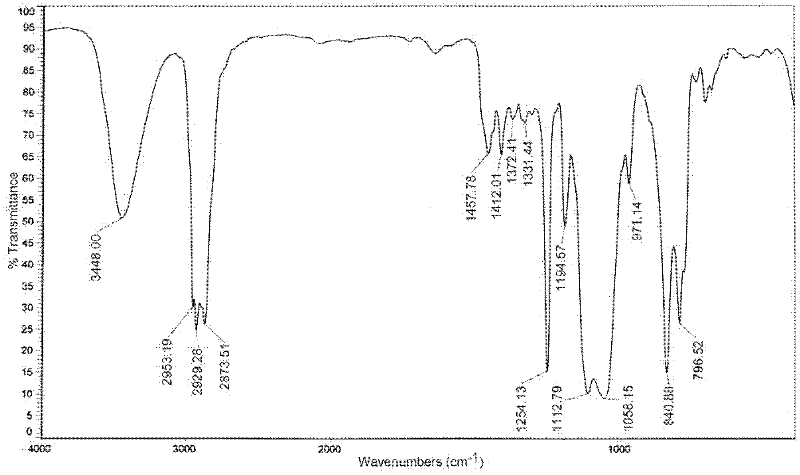
[0024] The product was characterized by infrared spectroscopy, as shown in the attached figure 1 The infrared spectrum shown. It can be seen from the map that the 1108cm -1 The stretching vibration absorption peak of the C-O-C bond appeared at 3051cm -1 The stretching vibration absorption peak of the C-H bond on the epoxy bond app...
Embodiment 2
[0031] Allyl glycidyl ether in the hydrosilylation reaction of Example 1 was replaced by 1,2-epoxy-8-nonene, 1,2-epoxy-8-nonene and tetramethyldisiloxane The ratio of the amount of alkane substance becomes 2.6: 1, and other reaction conditions are as described in embodiment 1, obtain the 1 of structure different from embodiment 1, the 3-diepoxy-terminated disiloxane, yield is 84% .
Embodiment 3
[0033] In the equilibrium reaction of embodiment 1, 1,3-bis(hydroxyethyl) hydroxyalkyl terminated disiloxane and D 4 The ratio of the amount of the substance becomes 1:9, and other reaction conditions are as described in Example 1, and the target compound double-terminal containing dihydroxyhydrocarbyl polysiloxane having an average molecular weight different from Example 1 of 3200 is obtained, and the yield 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com