Manidipine hydrochloride solid dispersion, preparation and preparation method of above
A technology of manidipine hydrochloride and solid dispersion, which is applied in the field of manidipine hydrochloride solid dispersion and preparation and its preparation, can solve the problems of long duration of action, achieve high dissolution rate, facilitate storage and high bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
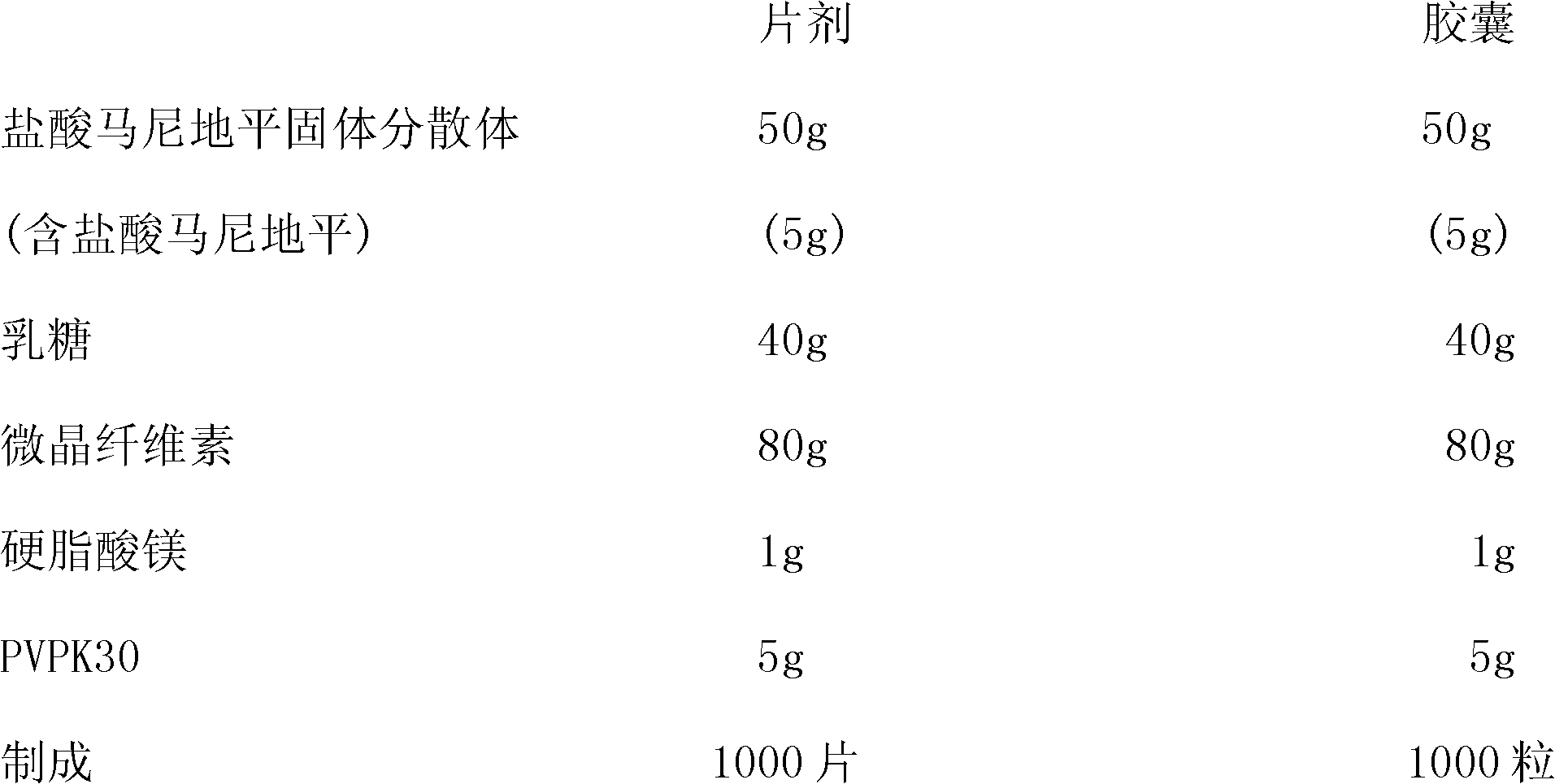
[0033] A solid dispersion of manidipine hydrochloride is composed of manidipine hydrochloride and dispersion medium PEG6000, the weight ratio of manidipine hydrochloride to PEG6000 is 10:90, wherein the average molecular weight of PEG6000 is 5500-7000.
[0034] Preparation method of manidipine hydrochloride solid dispersion: heat PEG6000 to 60-80°C to become liquid, add manidipine hydrochloride under continuous stirring, stir and disperse for 30 minutes, quickly pour it on a metal panel to form a thin layer of solid, and place it immediately Quench cooling below -20°C for 1 hour, take out and pulverize through a 100-mesh sieve to obtain a solid dispersion of manidipine hydrochloride.
Embodiment 2
[0036] A solid dispersion of manidipine hydrochloride is composed of manidipine hydrochloride and dispersion medium PEG6000, the weight ratio of manidipine hydrochloride to PEG6000 is 25:75, wherein the average molecular weight of PEG6000 is 5500-7000.
[0037] Preparation method of manidipine hydrochloride solid dispersion: heat PEG6000 to 60-80°C to become liquid, add manidipine hydrochloride under continuous stirring, stir and disperse for 50 minutes, quickly pour it on a metal panel to form a thin layer of solid, and place it immediately Cool at -30°C for 0.5 hour, take out and pulverize through an 80-mesh sieve to obtain a solid dispersion of manidipine hydrochloride.
Embodiment 3
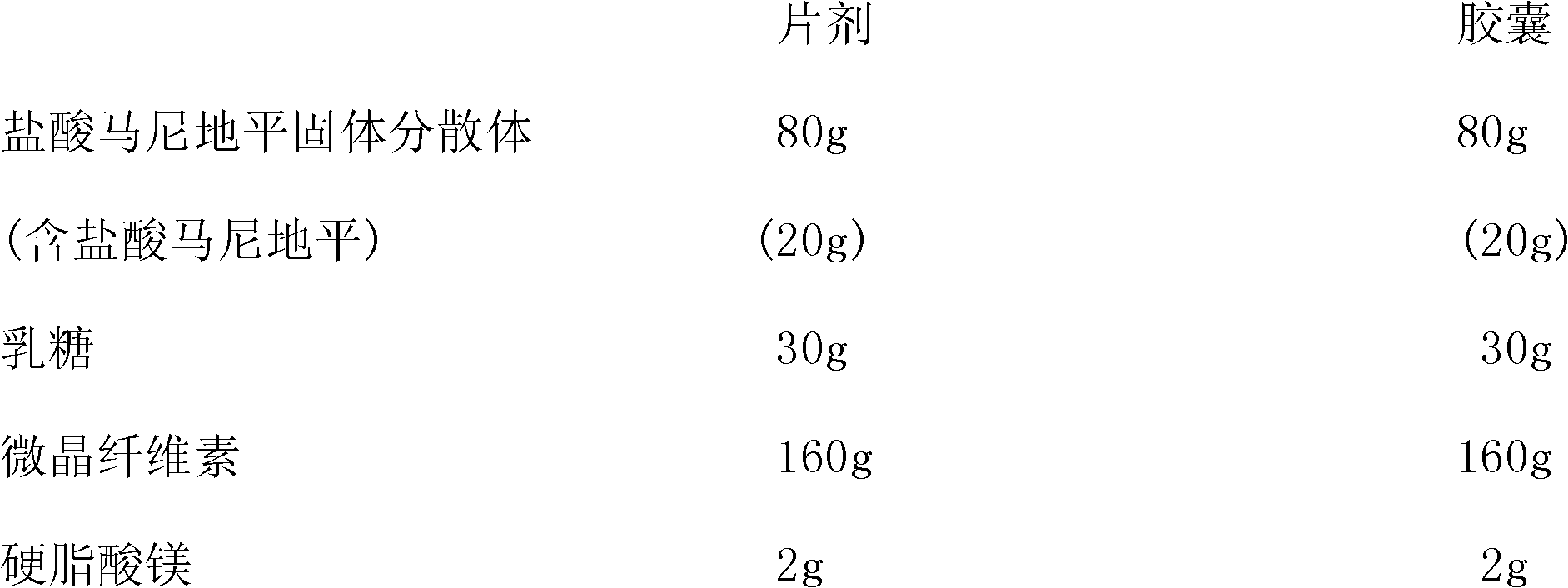
[0039] A solid dispersion of manidipine hydrochloride is composed of manidipine hydrochloride and dispersion medium PEG6000, the weight ratio of manidipine hydrochloride to PEG6000 is 40:60, and the average molecular weight of PEG6000 is 5500-7000.
[0040] Preparation method of manidipine hydrochloride solid dispersion: heat PEG6000 to 60-80°C to become liquid, add manidipine hydrochloride under continuous stirring, stir and disperse for 20 minutes, quickly pour it on a metal panel to form a thin layer of solid, and place it immediately Cool at -20°C for 2 hours, take out and pulverize through a 100-mesh sieve to obtain a solid dispersion of manidipine hydrochloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com