Medical preparation containing levocarnitine and dihydroxybenzenesulphonic acid salt and preparation method of medical preparation
A technology for dobesilate and solid pharmaceutical preparations, which is applied in the field of pharmaceutical preparations containing levocarnitine and dobesilate and its preparation, and can solve problems such as affecting production, difficulty in large-scale production, and large tablet discrepancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] In the preparation method of the pharmaceutical preparation of the present invention, the granulation may adopt dry granulation, wet granulation or spray granulation. Preferably, the granulation adopts wet granulation. Various granulation techniques are well known to those skilled in the art, for example, refer to the fourth edition of "Pharmacy" (Bi Dianzhou, People's Health Publishing House).
[0052] The third aspect of the present invention relates to the preparation of levocarnitine and dobesilate for the treatment and / or prevention of diseases affecting renal function such as primary nephritis, secondary nephritis, renal ischemia / reperfusion injury, Renal insufficiency (eg, chronic renal insufficiency), nephrotic syndrome, renal failure (eg, chronic renal failure), uremia and diabetic nephropathy, and complications from diseases affecting renal function such as cardiovascular disease (eg, myocardial Ischemia / reperfusion and chronic cardiac insufficiency) and diab...
Embodiment 1
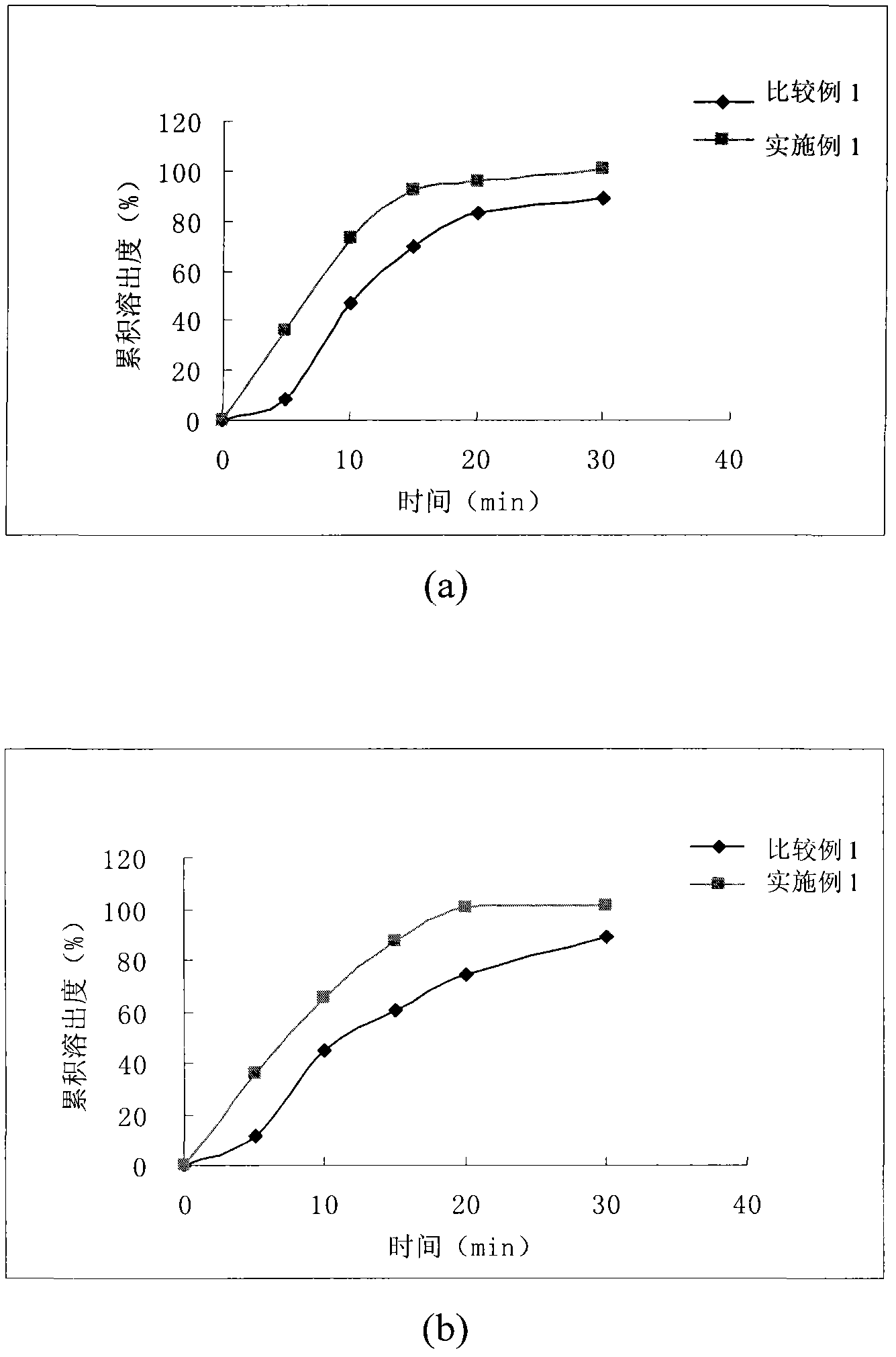
[0057] Example 1 - Tablets
[0058] formula:
[0059]
[0060]
[0061] Preparation Process:
[0062] Mix levocarnitine, microcrystalline cellulose and calcium sulfate, add an appropriate amount of binder to make a soft material, and dry at 50°C for 3 hours, as part A. Mix calcium dobesilate and microcrystalline cellulose, add an appropriate amount of binder to make a soft material, and dry at 50°C for 2 hours, as part B. Mix part A and part B, add crospovidone and low-substituted hydroxypropyl cellulose, mix well, then add magnesium stearate, mix well, and press into tablets.
Embodiment 2
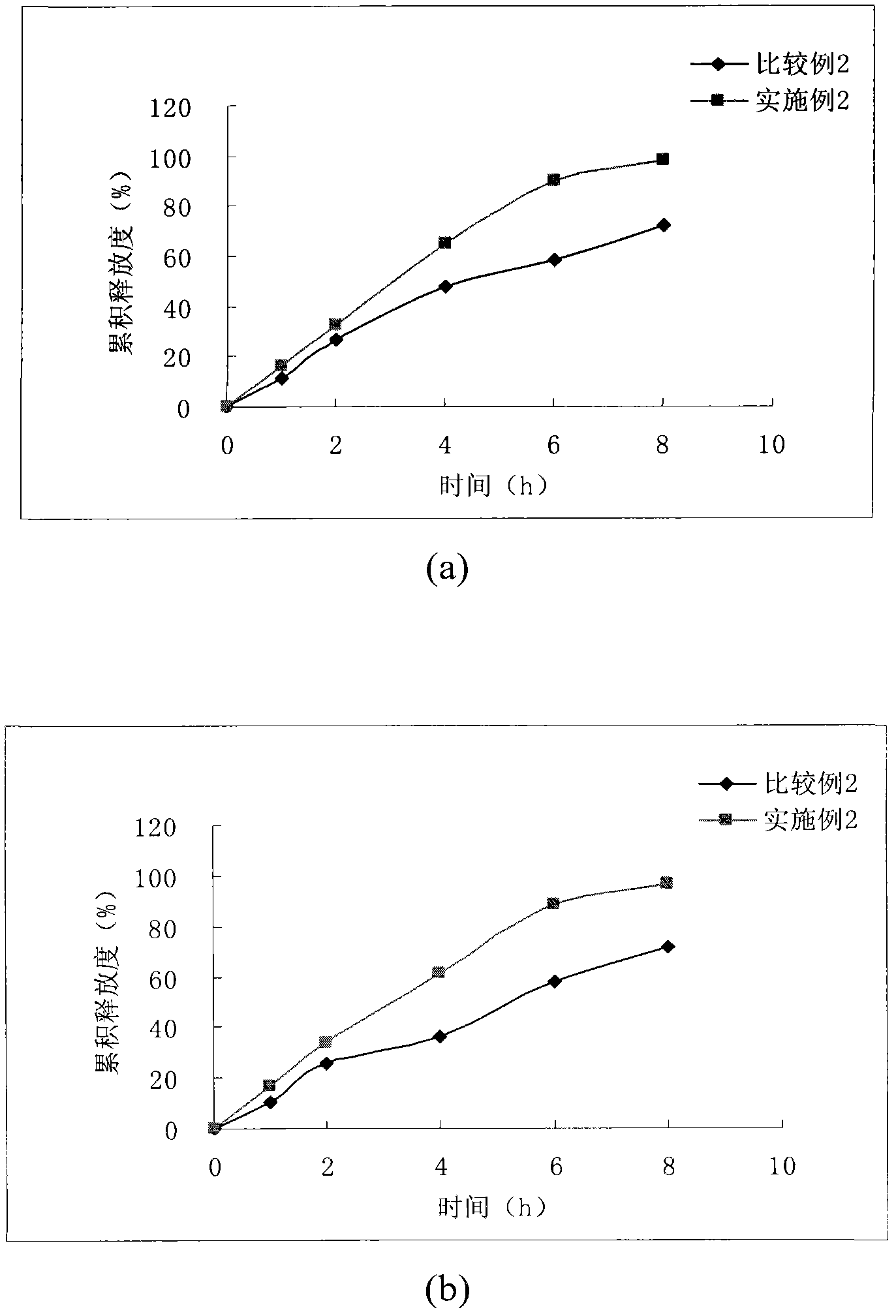
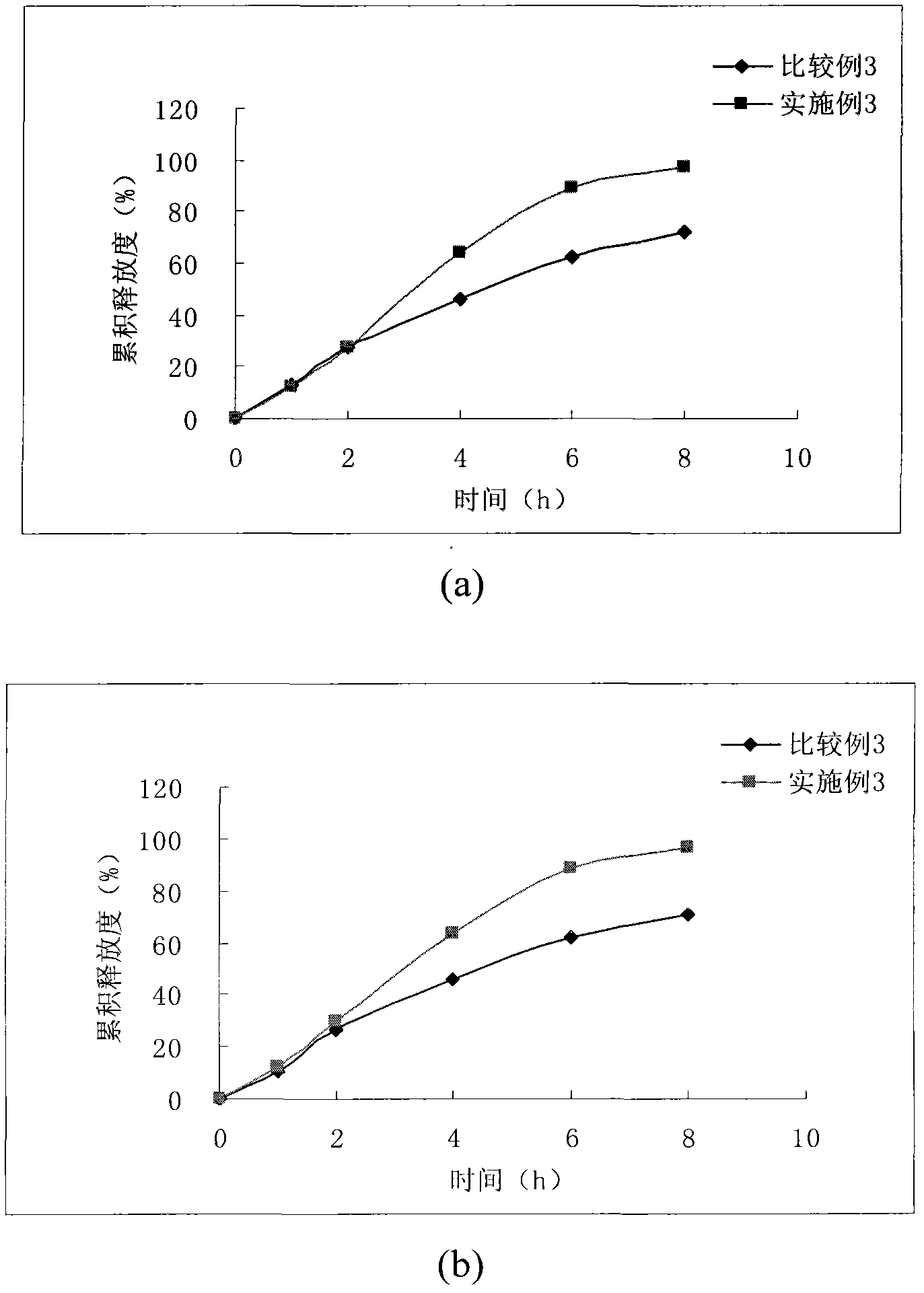
[0063] Example 2 - Sustained Release Tablets
[0064] formula:
[0065]
[0066] Preparation Process:
[0067] Mix levocarnitine, hydroxypropyl methylcellulose, microcrystalline cellulose and calcium sulfate, add an appropriate amount of binder to make a soft material, dry at 50°C for 3 hours, and use it as part A. Mix calcium dobesilate, hydroxypropyl methylcellulose and microcrystalline cellulose, add an appropriate amount of binder to make a soft material, dry at 50°C for 2 hours, and use it as part B. Mix part A and part B, add magnesium stearate, mix well, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com