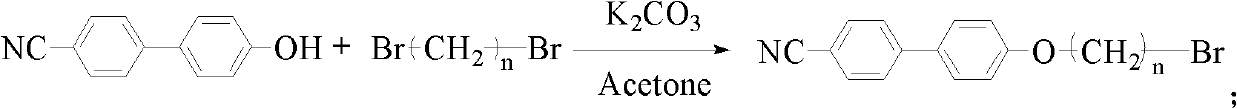Preparation and application of liquid crystal functionalized pyridine compound
A functional and compound technology, which is applied in organic chemistry, photosensitive equipment, semiconductor/solid-state device manufacturing, etc., can solve the problems of high additive cost, reduce electrolyte conductivity, increase electrolyte viscosity, etc., achieve less battery assembly procedures, and improve battery performance. Efficiency, cost reduction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
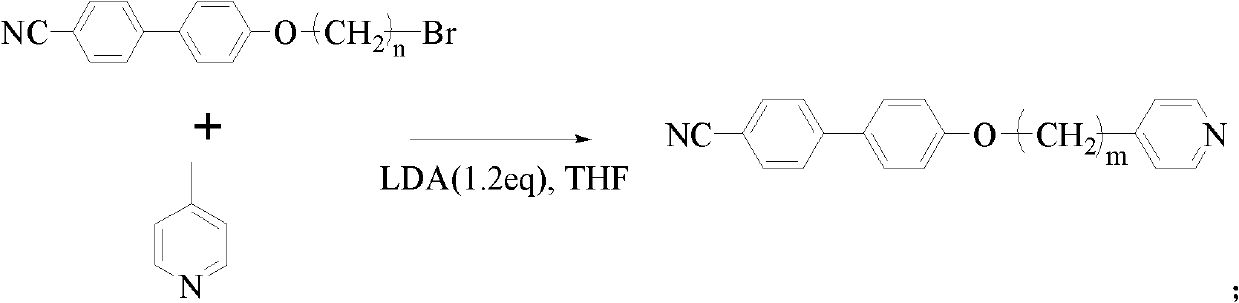
[0037] The synthesis of: 20mmol dibromoethane and 20mmol K 2 CO 3 were added to 10mmol In acetone (20mL) solution, N 2 Under protection, react at 70°C for 12h. Filtration, and removal of solvent gave a precipitate, which was recrystallized to give 1 HNMR (300MHz, CDCl 3 ): 7.67 (m, 4H), 7.54 (d, 2H), 7.02 (d, 2H), 4.35 (t, 2H), 3.67 (t, 2H). Get 1g (10.75mmol) 4-picoline and dissolve into THF (50mL), in N 2 Cooled to -30°C under protection, LDA (6.5mL, 12.90mmol) was slowly added dropwise, then stirred at -30°C for 0.5 hours, and finally 3.0g (10.0mmol) was added quickly, then stirred at -30°C for 0.5 hours, the solvent was removed under reduced pressure, and the product was obtained by passing through the column 1 HNMR (300MHz, CDCCl 3 ): 8.50(d, 2H), 7.83-7.85(dd, 4H), 7.70(d, 2H), 7.24(d, 2H), 7.06(d, 2H), 4.08(t, 2H), 2.78(t, 2H), 2.06(m, 2H).
Embodiment 2
[0039] The synthesis of: 20mmol dibromobutane and 20mmol K 2 CO 3 were added to 10mmol In acetone (20 mL) solution, under N2 protection, react at 70° C. for 12 h. Filtration, and removal of solvent gave a precipitate, which was recrystallized to give 1 HNMR (300MHz, CDCl 3 ): 7.66(m, 4H), 7.53(d, 2H), 6.99(d, 2H), 4.06(t, 2H), 3.51(t, 2H), 2.10(m, 2H), 1.98(m, 2H) . Get 1g (10.75mmol) 4-picoline and dissolve into THF (50mL), in N 2 Cooled to -30°C under protection, LDA (6.5mL, 12.90mmol) was slowly added dropwise, then stirred at -30°C for 0.5 hours, and finally 3.53g (10.3mmol) was added quickly, then stirred at -30°C for 0.5 hours, the solvent was removed under reduced pressure, and the product was obtained by passing through the column 1 HNMR (300MHz, CDCCl 3 ): 8.58(d, 2H), 7.86-7.84(dd, 4H), 7.69(d, 2H), 7.22(d, 2H), 7.06(d, 2H), 4.09(t, 2H), 2.69(t, 2H), 1.76 (m, 2H), 1.59-1.30 (m, 4H).
Embodiment 3
[0041] The synthesis of: 20mmol dibromohexane and 20mmol K 2 CO 3 were added to 10mmol In acetone (20 mL) solution, under N2 protection, react at 70° C. for 12 h. Filtration, and removal of solvent gave a precipitate, which was recrystallized to give 1 HNMR (300MHz, CDCl 3 ): 7.66(m, 4H), 7.53(d, 2H), 6.99(d, 2H), 4.01(t, 2H), 3.43(t, 2H), 1.91(m, 2H), 1.83(m, 2H) , 1.53 (s, 4H). Get 1g (10.75mmol) 4-picoline and dissolve into THF (50mL), in N 2 Cooled to -30°C under protection, LDA (6.5mL, 12.90mmol) was slowly added dropwise, then stirred at -30°C for 0.5 hours, and finally 3.82g (10.0mmol) was added quickly, then stirred at -30°C for 0.5 hours, the solvent was removed under reduced pressure, and the product was obtained by passing through the column 1 HNMR (300MHz, CDCCl 3 ): 8.50(d, 2H), 7.84-7.86(dd, 4H), 7.72(d, 2H), 7.27(d, 2H), 7.09(d, 2H), 4.06(t, 2H), 2.68(t, 2H), 1.76 (m, 2H), 1.58 (d, 2H), 1.27-1.45 (m, 6H).
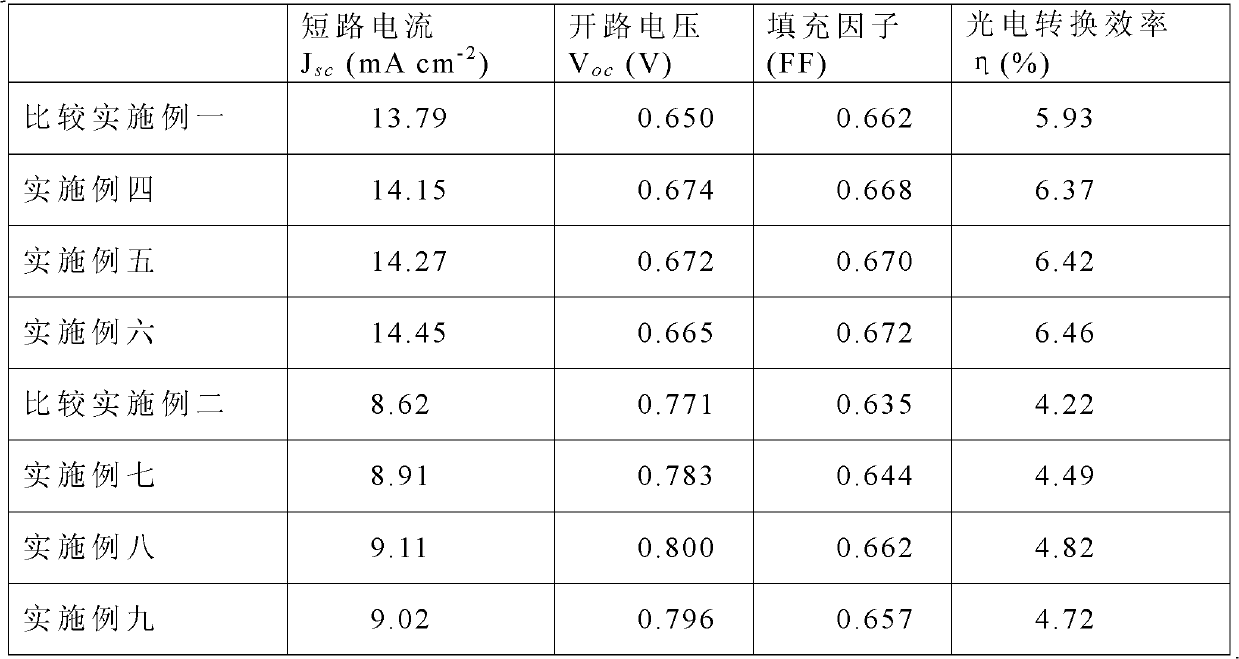
[0042] Embodiments 4 to 9 are specifi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com