Benzophenanthrene asymmetrical disk compound and preparation method thereof
A triphenylene and asymmetric technology, applied in the field of new triphenanthrene-like discotic compounds and their preparation, can solve the problems of few and few luminescent materials of derivatives of triphenanthrene, and achieve good organic electrophoresis The effect of luminescent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
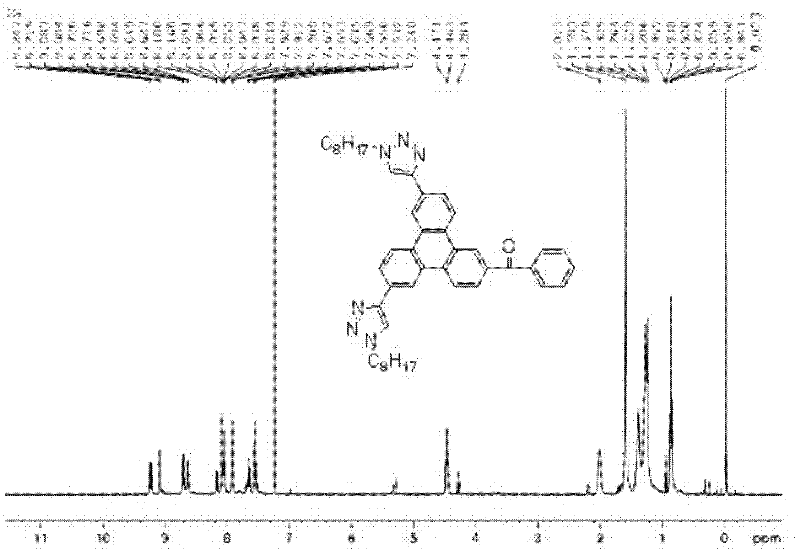
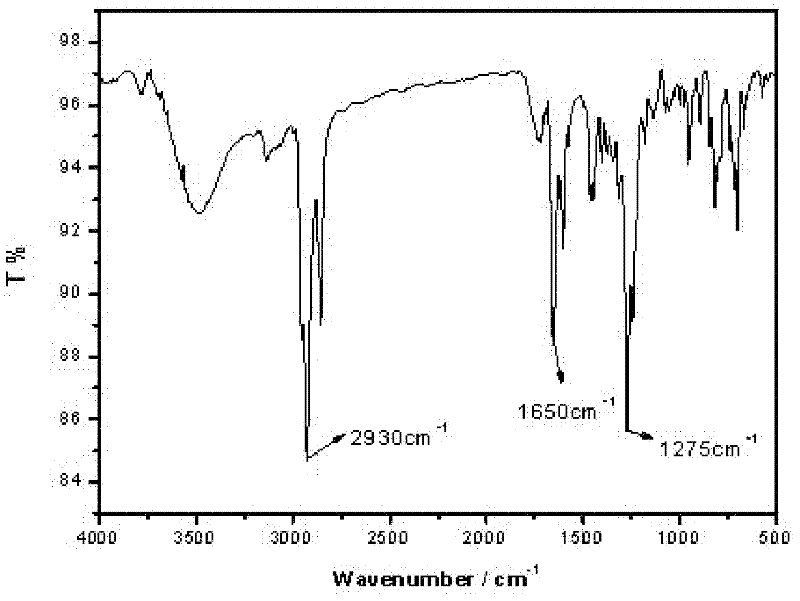
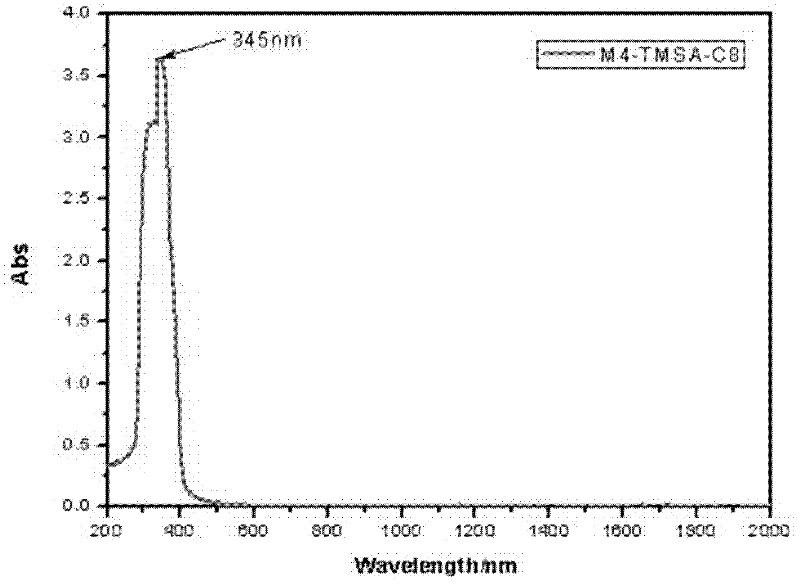
[0031] Embodiment one : Synthesis and Characterization of Compound 1
[0032]
[0033] 1: Friedel-Crafts acylation reaction of triphenylene: Dissolve 21.90mmol triphenylene (5.00g) in 60mL dichloromethane and put it in a 250mL one-necked bottle, add 23.00mmol p-benzoyl chloride (3.23g) , cooled to 0 degrees Celsius in an ice bath, gradually added 31.30mmol of aluminum oxide (4.17g); heated to reflux for 10 hours; the product was cooled and poured into 500mL of ice water and stirred overnight, extracted with dichloromethane, and the organic phase was passed through After drying and filtering with magnesium sulfate in water, it was purified with a silica gel column (the developer was a 1:1 mixed solvent of petroleum ether and dichloromethane) to obtain 3.25 g of benzoyl chloride-substituted triphenylene as a pale yellow solid, with a yield of 45%.
[0034]2: bromination reaction: 3.00mmol benzoyl chloride-substituted triphenylene (1.00g) obtained in step 1 was dissolved in ...
Embodiment 2
[0039] Embodiment two : Synthesis and Characterization of Compound 2
[0040]
[0041] 1: Friedel-Crafts acylation reaction of triphenylene: Dissolve 21.90mmol triphenylene (5.00g) in 60mL dichloromethane and put it in a 250mL one-necked bottle, add 23.00mmol p-benzoyl chloride (3.23g) , cooled to 0 degrees Celsius in an ice bath, gradually added 31.30mmol of aluminum oxide (4.17g); heated to reflux for 10 hours; the product was cooled and poured into 500mL of ice water and stirred overnight, extracted with dichloromethane, and the organic phase was passed through After drying and filtering with magnesium sulfate in water, it was purified with a silica gel column (the developer was a 1:1 mixed solvent of petroleum ether and dichloromethane) to obtain 3.25 g of benzoyl chloride-substituted triphenylene as a pale yellow solid, with a yield of 45%.
[0042] 2: bromination reaction: 3.00mmol benzoyl chloride-substituted triphenylene (1.00g) obtained in step 1 was dissolved in...
Embodiment 3
[0046] Embodiment three : Synthesis and Characterization of Compound 3
[0047]
[0048] 1: Friedel-Crafts acylation reaction of triphenylene: Dissolve 21.90mmol triphenylene (5.00g) in 60mL dichloromethane and put it in a 250mL one-necked bottle, add 23.00mmol p-benzoyl chloride (3.23g) , cooled to 0 degrees Celsius in an ice bath, gradually added 31.30mmol of aluminum oxide (4.17g); heated to reflux for 10 hours; the product was cooled and poured into 500mL of ice water and stirred overnight, extracted with dichloromethane, and the organic phase was passed through After drying and filtering with magnesium sulfate in water, it was purified with a silica gel column (the developer was a 1:1 mixed solvent of petroleum ether and dichloromethane) to obtain 3.25 g of benzoyl chloride-substituted triphenylene as a pale yellow solid, with a yield of 45%.
[0049] 2: bromination reaction: 3.00mmol benzoyl chloride-substituted triphenylene (1.00g) obtained in step 1 was dissolved ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com