ELISA (Enzyme-Linked Immuno Sorbent Assay) detection kit of animal rabies neutralizing antibody and application thereof
A kit and rabies technology, applied in the field of molecular biology and biology, can solve the problems of high detection cost, unfavorable promotion and use, maintenance of biological macromolecular activity, and low antigen purity, so as to avoid false negative results, low cost, and high sensitivity sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 kit
[0030] The preparation and coating of coated antigens adopts microcarrier suspension culture technology to culture Vero cells. After the cells grow into a single layer, they are inoculated with rabies virus CTN-1 strain virus according to a certain ratio, purchased from China Institute for the Control of Pharmaceutical and Biological Products, and cultured. and harvest virus fluid. The linear sucrose gradient zonal centrifugation, concentration, extraction and purification of virus fluid includes the following steps:
[0031] 1) After Vero cells grow into a monolayer on the microcarrier, inoculate rabies virus at a ratio of 0.03 multiplicity of infection (M.O.I), at a temperature of 33°C, a rotating speed of 40r / min, pH 7.6, dissolved oxygen 50%, and adding 0.05% bovine serum white The 199 medium of the protein is cultured under medium conditions, and the supernatant is harvested after 4-6 days of culture until the cells are comple...
Embodiment 2
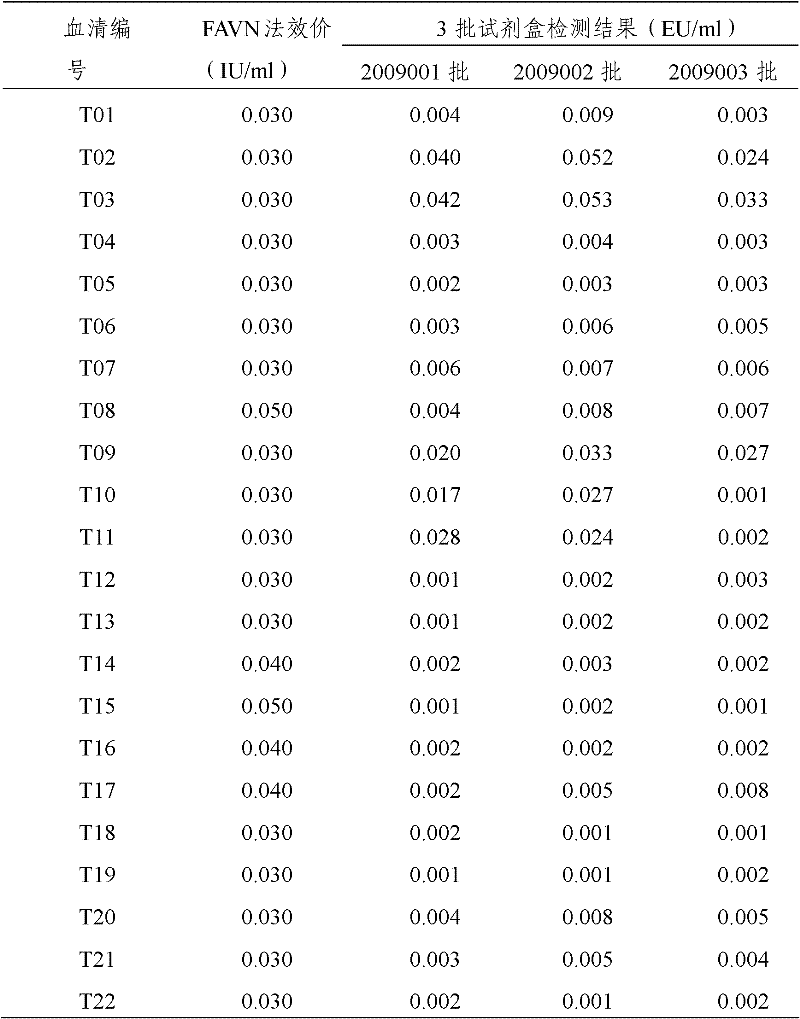
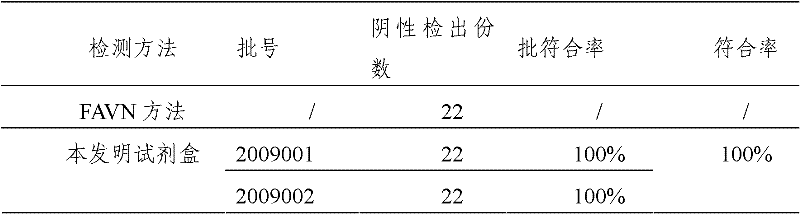
[0047] The assay procedure of rabies neutralizing antibody titer in embodiment 2 serum
[0048] Reconstitute positive serum and negative serum with 0.5ml sample diluent, and serially dilute to 1:100 by 10 times.
[0049] Reconstitute the standard serum with 0.5ml of sample diluent, obtain the standard curve according to the following dilution method, first dilute the standard serum to 0.4IU / ml, then dilute to 0.04IU / ml by 1:10, and perform 2 times on this basis Dilute to obtain the following gradient standard serum: 0.04IU / ml, 0.02IU / ml, 0.01IU / ml, 0.005IU / ml, 0.0025IU / ml, 0.00125IU / ml, 0.000625IU / ml.
[0050] Serum to be tested was serially diluted 10 times to 1:100.
[0051] Add diluted standard sera, negative sera, positive sera and samples to be tested with different gradient titers to the wells of the plate, 100ul / well, repeat in 2 wells. Incubate in a constant temperature incubator at 37°C for 30 minutes. Dilute the concentrated washing solution 20 times with deionize...
Embodiment 3
[0055] Embodiment 3 The formulation and characteristic evaluation of kit quality standard of the present invention
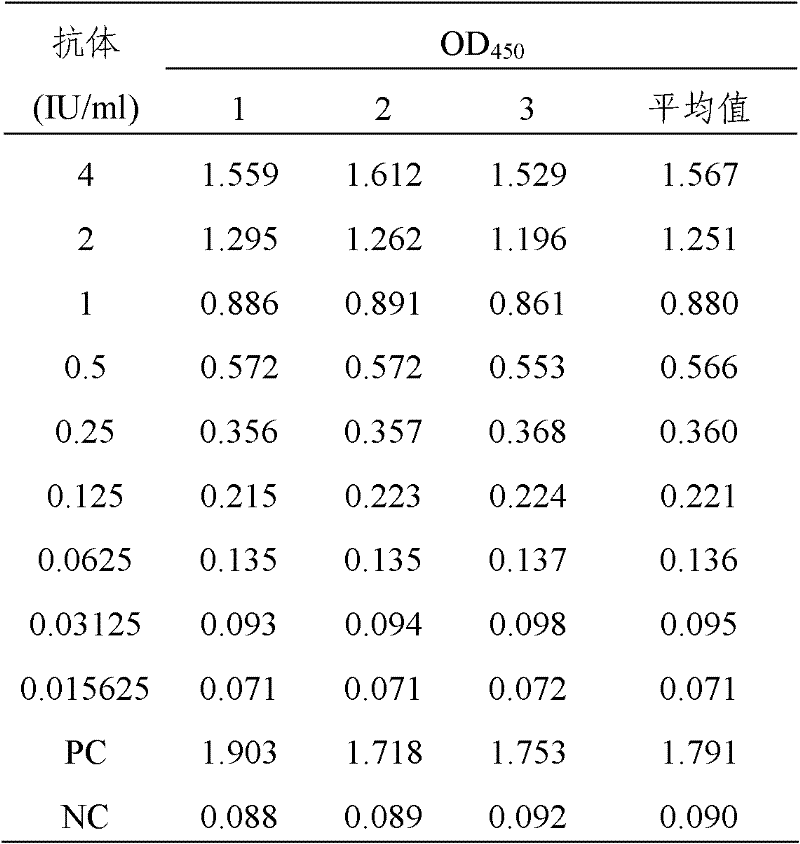
[0056] 1. Sensitivity: Dilute the OIE rabies positive serum (6.7IU / ml) with the sample diluent to 0.4IU / ml, then dilute it 10 times to 0.04IU / ml, then dilute it by 2 times, take 0.04, 0.02, 0.01, 0.005, 0.0025, 0.00125, 0.000625, 0.0003125, 0.00015625IU / ml titer serum, set up a negative control at the same time, repeat 2 wells for each dilution, follow the kit operation steps, get higher than the negative control serum OD value corresponding The serum antibody titer of the kit is the minimum detection limit of the rabies virus antibody of the kit, and the results are shown in Table 1.
[0057] Table 1 Test results of OIE rabies positive serum kit after doubling dilution
[0058]
[0059] Note: PC is positive control serum, NC is negative control serum.
[0060] It can be seen from the above table that when the OIE rabies positive serum is 0.015625IU / ml, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com