Sustained release injection preparation and preparation method and application thereof
A technology for sustained release and injection preparations, which is applied to the sustained release injection preparations of psychotropic drugs. The field of preparation of the sustained release injection preparations can solve the problem of unsatisfactory drug sustained release effects and stability, drug body and preparation stability Adverse effects, reducing the shelf life of drugs, drug safety and other issues, to achieve the effect of improving physical stability, facilitating industrial production, and improving chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
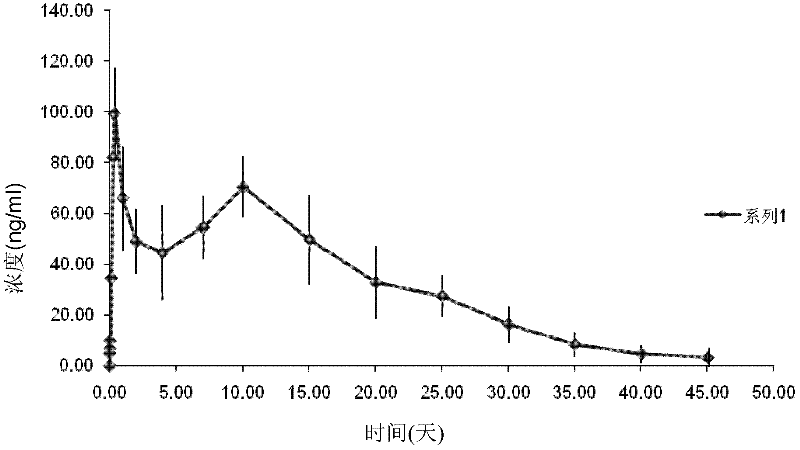
[0033] Embodiment 1 prepares aripiprazole polyethylene glycol injection (100mg / ml)
[0034] First, add 50 g of aripiprazole and 500 ml of PEG400 into a horizontal grinding machine (SWZX-0.4 horizontal grinding machine, Shanghai Shihe Electromechanical Equipment Co., Ltd.), and first grind at 20 Hz for 3 to 5 minutes to crush large particles. The medicine and the polyethylene glycol are fully mixed, then the grinding speed is increased, and the grinding is continued for 30 minutes at 45 Hz.
[0035] Then it was added to a high-pressure counter-jet homogenizer (Nano DeBEE), and the inlet temperature was kept at about 40° C., and the high-pressure homogenization cycle was performed at 500 bar for 3 times to obtain a primary suspension, which was collected for use. Then the inlet temperature was adjusted to 10°C, the primary suspension was homogenized at 1500 bar, the liquid was circulated 6 times, and the medicinal solution was collected.
[0036] The particle size distribution ...
Embodiment 2
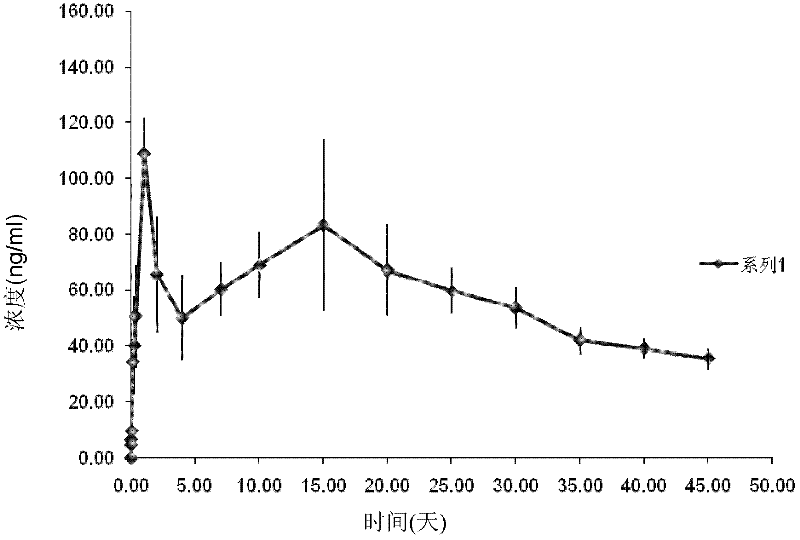
[0037] Example 2 Preparation of Aripiprazole Polyethylene Glycol Injection (200mg / ml)
[0038] First, add 100 g of aripiprazole and 500 ml of PEG400 into a horizontal grinding machine (SWZX-0.4 horizontal grinding machine, Shanghai Shihe Electromechanical Equipment Co., Ltd.), and first grind at 20 Hz for 3 to 5 minutes to crush large particles. , so that the medicine and polyethylene glycol are fully mixed, then the grinding speed is increased, and the grinding is continued for 40 minutes at 45 Hz.
[0039] Then, it was added to a high-pressure counter-jet homogenizer (Nano DeBEE), and the inlet temperature was kept at about 40° C., and the high-pressure homogenization cycle was performed at 500 bar for 3 times to obtain a primary suspension, which was collected for use. Then the inlet temperature was adjusted to 10°C, the primary suspension was homogenized at 1500 bar, the liquid was circulated 6 times, and the medicinal solution was collected.
[0040] The particle size di...
Embodiment 3
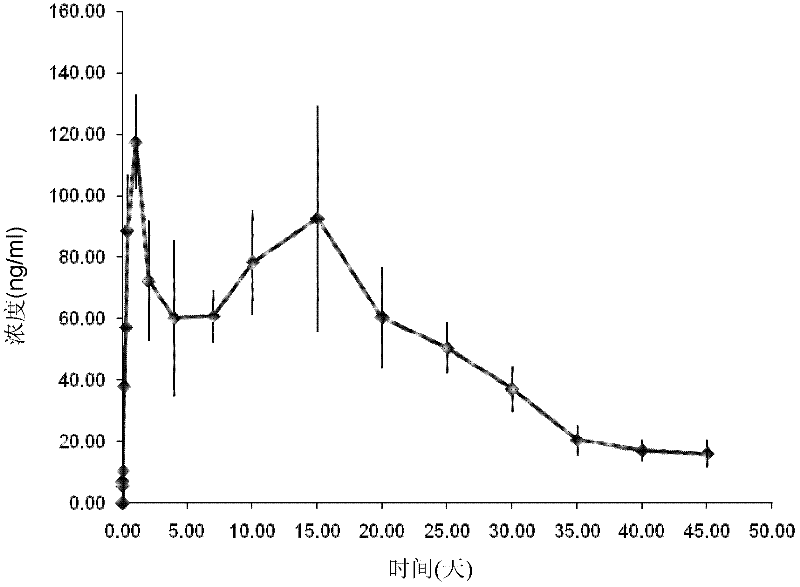
[0041] Embodiment 3 prepares aripiprazole soybean oil injection (200mg / ml)
[0042] First, aripiprazole was pulverized to a D4,3 of 3.45 μm using a jet pulverizer (MX-50 supersonic jet pulverizer, Yixing Juneng Superfine Pulverization Equipment Co., Ltd.).
[0043] Then, 100 g of aripiprazole and 500 ml of soybean oil for injection were initially dispersed (20 Hz for 3 minutes) with a horizontal grinding machine (SWZX-0.4 type horizontal grinding machine, Shanghai Shihe Electromechanical Equipment Co., Ltd.), and then 40 Hertz mill for 20 minutes.
[0044]The particle size distribution of the product was evaluated with a MASTERSIZER 2000 laser particle size analyzer. The product has the following characteristics: the volume average particle size D4,3 is 2.13 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com