Application of oridonin in preparing activator of tumor-suppressor protein FBW7
A technology of Rubescensine A and tumor suppressor protein, which is applied in the fields of medicine, biochemistry and cell biology, can solve problems such as no discovery, and achieve the effects of low human toxicity, easy preparation and strong applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
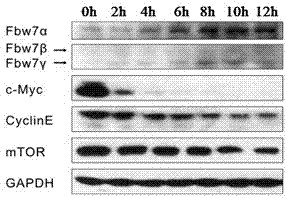
[0025] Example 1 Rubescensine A activates FBW7 protein in K562 cells and promotes ubiquitin-proteasome degradation of its substrate protein
[0026] K562 cells were divided into 3×10 5 / mL density was inoculated in 24-well plates, and Rubescensin A was added the next day to make the final concentration 20 μmol / L. Subsequently, the cells were collected at 0, 2, 4, 6, 8, 10, and 12 hours, and the proteins were lysed and detected by immunoblotting for FBW7 and its substrate proteins: c-Myc, CyclinE (cyclin E), mTOR (mammalian Expression of animal target of rapamycin). See the experimental results figure 1 , the protein levels of the three isoforms of FBW7 gradually increased over time under the induction of Rubescensin A, while the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as an internal control did not change. Oridonin can not only activate FBW7 in a short period of time, but also the effect will be more significant as the induction time prolongs. At...
Embodiment 2
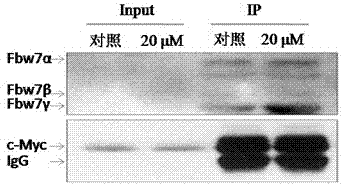
[0028] Example 2 Oridonin A promotes the binding of FBW7 to its substrate protein
[0029] We induced K562 cells with 20 μmol / L oridonin, collected the cells and lysed the protein after 2 hours. Cell lysates were co-immunoprecipitated using c-Myc-specific antibodies, and the precipitated protein complexes were immunoblotted to detect FBW7 in the protein complexes. from image 3 It can be seen from the results that Rubescensin A significantly increased FBW7 in the c-Myc immunoprecipitated protein complex, that is, Rubescensin A significantly enhanced the binding ability of FBW7 to the substrate protein c-Myc .
Embodiment 3
[0030] Example 3 The growth inhibition and apoptosis induction effect of oridonin on K562 cells
[0031] We divided K562 cells into 3 × 10 5 The cells were inoculated in 96-well plates at a density of / mL, and treated with 5-80 μmol / L oridonin for 24 hours the next day (with DMSO as the control), and then the cell viability was detected by MTT. See the experimental results Figure 4 A, The cell viability was not significantly affected by the low concentration of Rubescensin A treatment, and the concentration above 20 μmol / L could significantly reduce the survival rate of K562 cells. The inhibitory effect of oridonin on K562 cells was positively correlated with drug dosage.
[0032] K562 cells were induced with 20 μmol / L oridonin for 24 hours (DMSO was used as the control), the collected cells were washed once with PBS, and stained with DAPI for 5 minutes. Examined under a fluorescent microscope, most of the cells undergo apoptosis, which can be deeply stained by DAPI, and n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com