Preparation method and application of phthalic acid diester (2-propyl group heptanol)
A technology of phthalic acid di- and phthalic anhydride is applied in the field of preparation of phthalic acid diesters and achieves the effects of good compatibility, good thermal aging performance and good anti-fogging performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
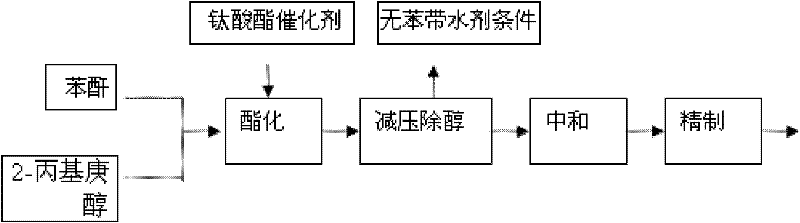
[0022] at 25M 3 Put phthalic anhydride and 2-propylheptanol into the stainless steel reaction kettle. When feeding, the molar ratio of phthalic anhydride to 2-propylheptanol is 1:2.6. Stir and heat up. When the temperature rises to 130°C, stir at constant temperature for 50 minutes and continue to heat up. After reaching 180°C, add catalyst titanate, continue to react at 210°C for 5 hours, remove alcohol under reduced pressure, neutralize, separate the water phase and refine to obtain the product.
Embodiment 2
[0024] at 25M 3 Put phthalic anhydride and 2-propylheptanol into the stainless steel reaction kettle. When feeding, the molar ratio of phthalic anhydride to 2-propylheptanol is 1:3. Stir and heat up. When the temperature rises to 130°C, stir at constant temperature for 50 minutes and continue to heat up. To 180°C, add catalyst titanate, continue to react at 180°C-210°C for 3 hours, remove alcohol under reduced pressure, neutralize with 5% sodium hydroxide solution, without washing with water, and dehydrate under reduced pressure to obtain the product.
[0025] The obtained product quality index and the energy consumption index required for production are as follows:
[0026] Table 1 DPHP quality indicators
[0027] Indicator name Index value color, APHA ≤10 Acid value, mg KOH / g ≤0.02 Moisture, % ≤0.02 Viscosity, mPas ≤125 Specific gravity (20℃), g / cm3 0.962±0.003 Refractive index(25℃) 1.484±0.003 Ester content, ...
Embodiment 3
[0035] Combined with the excellent performance of the DPHP of the present invention, it is applied to UL wire and cable, and experiments are carried out at 60°C, 75°C, 80°C, and 90°C respectively, and the data obtained are as follows in Table 4:
[0036] Table 4 Specific application of DPHP in UL wire and cable
[0037] UL level number of days temperature(℃) 60 7 100 75 10 100 80 7 114 90 7 122
[0038] *UL: Underwriter Laboratories (US)
[0039]
[0040] Conclusion: 1. 60°C, 75°C
[0041] Interchangeable with DIDP in all insulation and jacket formulations using DIDP (UL Recognized)
[0042] 2. 80°C, 90°C
[0043] Can be mixed with other plasticizers, such as TOTM, etc.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com