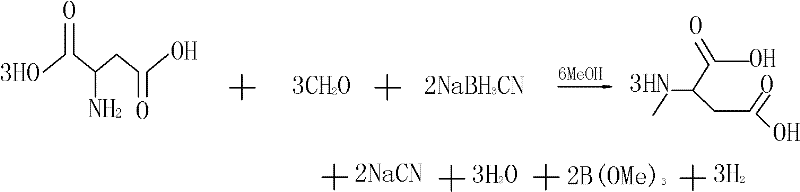Method for synthesizing N-methyl-D-aspartate
A technology for aspartic acid and synthesis methods, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as complex reactions and difficult separation of products, and achieve the effect of easy separation and extraction and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0009] A kind of synthetic method of N-methyl-D-aspartic acid, comprises the following steps:
[0010] a) in methanol solvent, add D-aspartic acid, paraformaldehyde and reducing agent, carry out reaction;
[0011] b) After the reaction, the white precipitate produced by the reaction is filtered out, and then washed with methanol to obtain the product N-methyl-D-aspartic acid.
[0012] The present invention adopts reductive amination method, one-step reaction to obtain the product, the process is simple, and the product is easy to separate and extract.
[0013] The reaction formula is as follows:
[0014]
[0015] The invention uses D-aspartic acid as raw material, reacts excess paraformaldehyde and reducing agent in methanol solvent. After the reaction, a white precipitate appeared, which was separated and washed with methanol to obtain the product N-methyl-D-aspartic acid.
[0016] The filtrate obtained by filtering and the washing liquid obtained by washing in the step...
Embodiment 1
[0023] 13.3g of D-aspartic acid, 1.995g of paraformaldehyde and 6.65g of sodium cyanoborohydride were respectively added into a three-necked flask containing 133g of methanol solvent, and the reaction was stirred at room temperature at pH 6.0-8.0 for 18 hours. After the reaction was completed, the white precipitate was filtered and washed twice with 20-30ml of methanol to obtain 5.5g of the product. Collect the filtrate and washings, pass through a chromatographic column (φ20×500) equipped with 100g silica gel (200~300 mesh) at a flow rate of 5ml / min, use a chloroform-methanol-water system with a ratio of 6:4:1 300ml was eluted, and the collected eluent was concentrated under reduced pressure to crystallize and then dried to obtain 2.3g of the product, with a total yield of 53%. Product optical rotation: [α]D 20 =-28.32° (c=1,6N HCl), mp 187°C.
Embodiment 2
[0025] 13.3g of D-aspartic acid, 2.97g of paraformaldehyde and 9.5g of sodium cyanoborohydride were respectively added into a three-necked flask filled with 240.3g of methanol solvent, and the reaction was stirred at room temperature at pH 6.0-8.0 for 18 hours. After the reaction was completed, the white precipitate was filtered and washed twice with 20-30ml of methanol to obtain 9.2g of the product. The filtrate and methanol washings were carried out in the same manner as in Example 1 to obtain 3.7 g of the product. The total yield is 87.8%. Product optical rotation: [α]D 20 =-28.85° (c=1,6N HCl), mp 188°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com