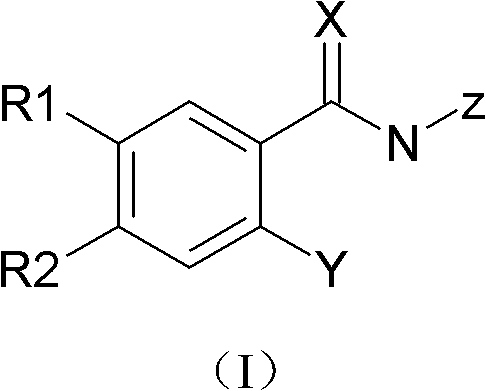
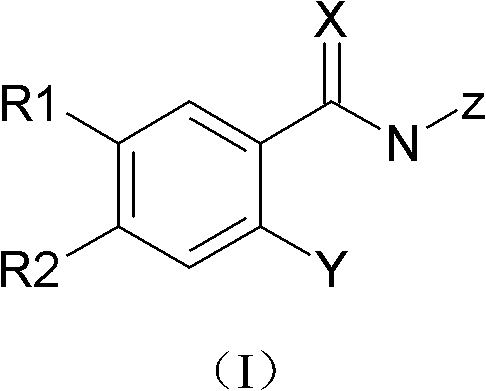
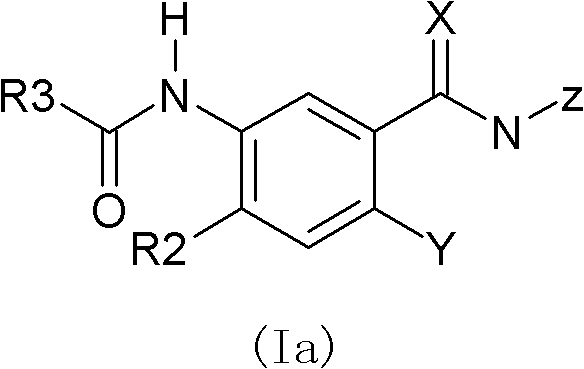
Substituted benzamide compounds and preparation method and application thereof
A technology of benzamides and compounds, applied in the field of application in the treatment of cancer, can solve problems such as proliferative diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1: N-(3-bromophenyl)-2-hydroxyl-5-bromoacetamidobenzamide
[0105]
[0106] Preparation of N-(3-bromophenyl)-2-acetoxy-5-nitrobenzamide:
[0107] Take 5-nitrosalicylic acid (1.83g, 10mmol) and acetic anhydride (4.08g, 40mmol) in a flask, add 2 drops of concentrated sulfuric acid dropwise, gradually heat up to 85-90°C under slow stirring, and react for 5h. After cooling and filtering, the solid was washed with cold water, a small amount of saturated NaHCO3 solution, and water, and dried to obtain 1.75 g of 5-nitro-2-acetoxybenzoic acid light yellow solid powder with a yield of 77.8% and a melting point of 161-162°C. Take 5-nitro-2-acetoxybenzoic acid (1.125g, 5mmol), add appropriate amount of SOCl 2 , heated to reflux for 3h, evaporated under reduced pressure to remove excess SOCl 2 , to obtain an oily substance, add an appropriate amount of ethyl acetate to dissolve, slowly add 3-chloroaniline (0.86g, 5mmol) and an appropriate amount of triethylamine in eth...
Embodiment 2
[0115] Example 2: N-(3-bromophenyl)-2-hydroxyl-5-chloroacetamidobenzamide.
[0116]
[0117] Preparation of N-(3-bromophenyl)-2-acetoxy-5-chloroacetamidobenzamide:
[0118] Take N-(3-bromophenyl)-2-acetoxy-5-aminobenzamide (0.524g, 1.5mmol), add an appropriate amount of ethyl acetate to dissolve, add a few drops of triethylamine, and place in an ice-water bath chloroacetyl chloride (0.17g, 1.5mmol) was slowly added dropwise, and the reaction solution became cloudy. After the dropwise addition, it was reacted at room temperature for 1 hour, and the precipitate was filtered off. NaCl solution, followed by anhydrous NaCl 2 SO 4 After drying, the solvent was distilled off under reduced pressure, and recrystallized from ethyl acetate-petroleum ether to obtain 0.419 g of khaki solid powder with a yield of 65.6%.
[0119] Preparation of N-(3-bromophenyl)-2-hydroxyl-5-chloroacetamidobenzamide:
[0120] Take N-(3-bromophenyl)-2-acetoxy-5-chloroacetamidobenzamide (0.524g, 1.5mmol...
Embodiment 3
[0122] Example 3: N-((S)-1-phenylethyl)-2-hydroxyl-5-bromoacetamidobenzamide.
[0123]
[0124] Preparation of N-((S)-1-phenethyl)-2-acetoxy-5-nitrobenzamide:
[0125] Take 5-nitro-2-acetoxybenzoic acid (1.21g, 10mmol), add appropriate amount of SOCl 2 , heated to reflux for 3h, evaporated under reduced pressure to remove excess SOCl 2 , to obtain an oily substance, add an appropriate amount of ethyl acetate to dissolve, and slowly add (S)-1 phenylethylamine (1.21g, 10mmol) and an appropriate amount of triethylamine in ethyl acetate solution dropwise under ice-water bath, and dropwise Afterwards, react at room temperature for 1 h, filter, and the filtrate is washed with 1mol / l hydrochloric acid, 10% sodium bicarbonate solution, saturated NaCl solution, and then washed with anhydrous Na 2 SO 4 After drying, the solvent was distilled off under reduced pressure, and the resultant was recrystallized from ethyl acetate-petroleum ether to obtain 2.79 g of white needle crystals...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com