Method for performing electrolytic refining on lead bullion
An electrolytic refining and crude lead technology, applied in the field of crude lead refining, can solve the problems of unpleasant smell, no practical progress in research, low electrolysis temperature, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
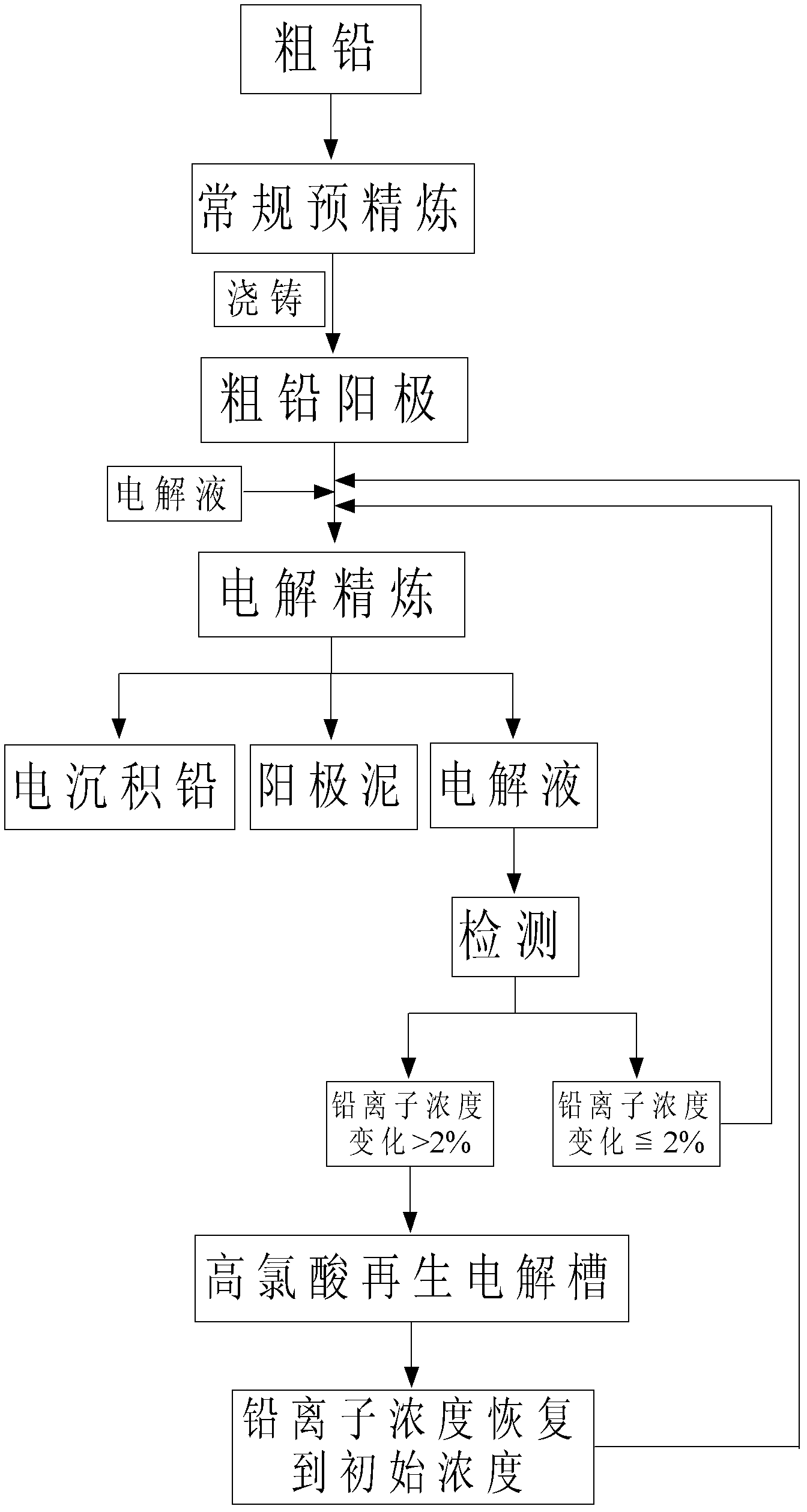
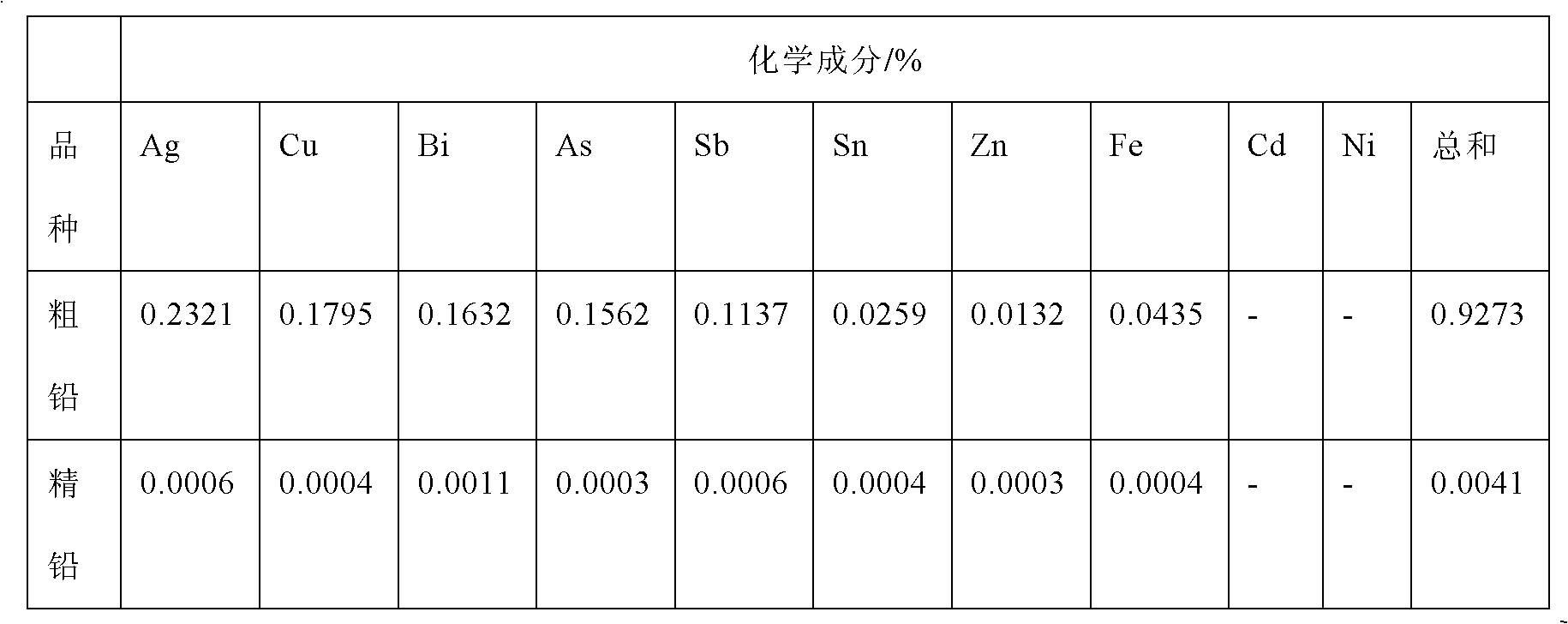
[0041] The common crude lead in the market is pre-refined by conventional fire method to make the crude lead content reach 99%. Melt 1kg of crude lead and cast it into 8*11*1 (width*height*thickness) cm 3 The anode plate with a size of 8*11*0.05 (width*height*thickness) cm 3 The electrodeposited lead is used as the initial electrode sheet, and the distance between the anode and the cathode is controlled to be 2.6-3.0cm, so that they are placed in the electrolytic cell in parallel.
[0042] (3) Concentration of 300ml is 2.5M HClO 4 and 0.3M Pb(ClO 4 ) 2 Mix the solution, then add boric acid and bone glue, so that boric acid 0.5g / L and bone glue 2.5g / L are used as two kinds of additives in the electrodeposition process.
[0043] (4) Inject the mixed electrolyte solution obtained in the process of (3) into the electrolytic cell, and after the temperature of the electrolyte solution stabilizes at 35°C, connect the power supply and start electrolysis in stages. In the first st...
Embodiment 2
[0050] Continue to take the same thick lead anode and electrodeposited lead as the initial electrode sheet in Example 1, and control the distance between the anode and the cathode to be 2.6-3.0cm, so that they are placed in the electrolytic cell in parallel.
[0051] (3) Concentration of 300ml is 2.2M HClO 4 and 0.35M Pb(ClO 4 ) 2 Mix the solution, then add 0.02g / L p-methoxybenzoic acid and 0.5g / L boric acid, 2.5g / L bone glue and 2.0g / L sodium lignosulfonate to add Additive 1 and Additive 2, respectively.
[0052] (4) Inject the mixed electrolyte solution obtained in the process of (3) into the electrolytic tank, and after the temperature of the electrolyte solution stabilizes at 45°C, connect the power supply and start electrolysis in stages. In the first stage of electrolysis, the potentiodynamic electrolysis mode was first adopted, in which the scan rate was 1.5 mV / min, and the scan interval was 0-120 mV. After 80 minutes of potentiodynamic electrolysis, enter the second...
Embodiment 3
[0060] Continue to take the same thick lead anode and electrodeposited lead as the initial electrode sheet in Example 1, and control the distance between the anode and the cathode to be 2.6-3.0cm, so that they are placed in the electrolytic cell in parallel.
[0061] (3) The configuration concentration is 1.6mol / L HClO 4 and 0.30mol / L of Pb(ClO 4 ) 2 Mix solution 300ml, then add 0.5g / L boric acid and 0.5g / L ammonium dihydrogen phosphate as additive 1, 2.0g / L bone glue and 0.05g / L coumarin as electrodeposition process Additive 2.
[0062] (4) Inject the mixed electrolyte solution obtained in the process of (3) into the electrolytic cell, and after the temperature of the electrolyte solution stabilizes at 55°C, connect the power supply and start electrolysis in stages. In the first stage of electrolysis, the potentiodynamic electrolysis mode was first adopted, in which the scan rate was 30 mV / min, and the scan interval was 0-120 mV. After 4 minutes of potentiodynamic electro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com