Surface modification method of lithium iron phosphate cathode material
A technology of lithium iron phosphate and cathode material, applied in battery electrodes, electrical components, circuits, etc., can solve problems such as cumbersome surface coating technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
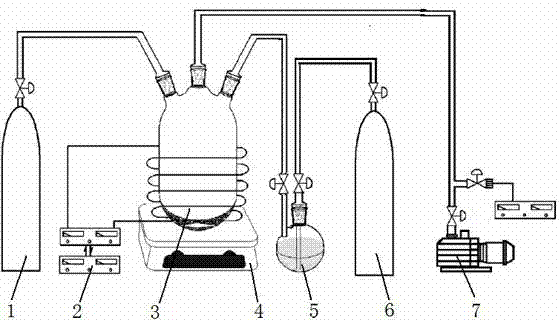
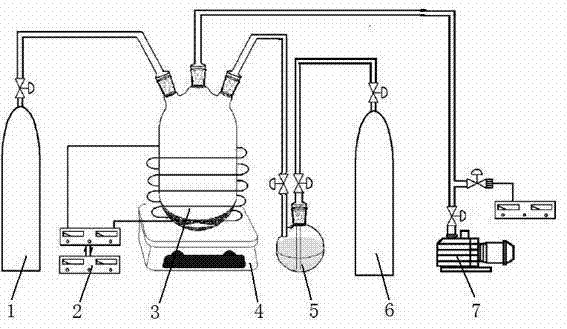
[0037] Weigh 5 grams of lithium iron phosphate cathode material powder into the plasma reactor 3, stir it in real time with a water bath magnetic stirrer 4, and control the temperature to 49-51°C. Pass the working gas N into the reactor 3 at a flow rate of 10 ml / min 2 , while passing carrier gas H into the organic monomer storage bottle 5 at a flow rate of 10 ml / min 2 , Liquid pyrrole is housed in the organic monomer storage bottle 5 . Pyrrole vapor and carrier gas H 2 into the plasma reactor 3 together. The reactor is continuously evacuated with a mechanical vacuum pump 7 to maintain a vacuum degree of 1-2 Pa in the reactor 3. Turn on the radio frequency power source 2, adjust the voltage to 30V, and the current to 20mA to generate plasma discharge in the reactor 3. The pyrrole monomer undergoes a polymerization reaction under the action of the plasma to generate a conductive polymer polypyrrole, which is coated on the surface of the lithium iron phosphate powder material...
Embodiment 2
[0040] Weighed 10 grams of lithium iron phosphate cathode material powder and put it into the plasma reactor 3, and stirred it in real time with the magnetic stirrer 4, and the temperature was normal temperature 25°C. Pass into the working gas Ar in the reactor 3 with the flow rate of 5 milliliters / minute, pass into the carrier gas He in the organic monomer storage bottle 5 with the flow rate of 5 milliliters / minute simultaneously, liquid state is housed in the organic monomer storage bottle 5 Thiophene. The thiophene vapor enters the plasma reactor 3 together with the carrier gas He. The reactor 3 is continuously evacuated with a mechanical vacuum pump 7 to maintain a vacuum degree of 0.1-0.2 Pa in the reactor 3. Turn on the radio frequency power source 2, adjust the voltage to 10V, and the current to 10mA to generate plasma discharge in the reactor 3. The thiophene monomer undergoes a polymerization reaction under the action of the plasma to generate a conductive polymer p...
Embodiment 3
[0043] Weigh 15 grams of lithium iron phosphate cathode material powder into the plasma reactor 2, stir it in real time with an oil bath magnetic stirrer 4, and control the temperature to 149-151°C. Pass the working gas N into the reactor 3 at a flow rate of 100 ml / min 2 , while passing carrier gas H into the organic monomer storage bottle 5 at a flow rate of 100 ml / min 2 , Liquid aniline is housed in the organic monomer storage bottle 5 . Aniline vapor and carrier gas H 2 into the plasma reactor 3 together. The reactor 3 is continuously evacuated with a mechanical vacuum pump 7 to maintain a vacuum degree of 10-20 Pa in the reactor 3. Turn on the radio frequency power source 2, adjust the voltage to 100V, and the current to 100mA to generate plasma discharge in the reactor. The aniline monomer undergoes a polymerization reaction under the action of the plasma to generate a conductive polymer polyaniline, which is coated on the surface of the lithium iron phosphate powder ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Reverse discharge specific capacity | aaaaa | aaaaa |
| Reverse discharge specific capacity | aaaaa | aaaaa |
| Reverse discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com