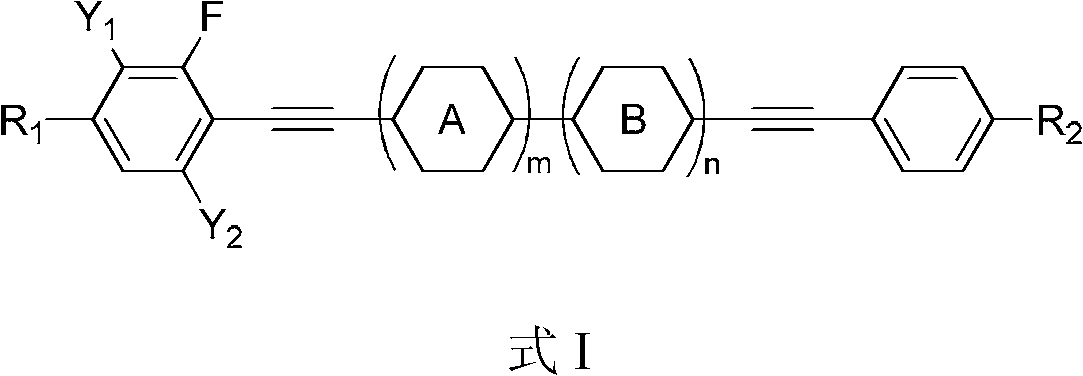
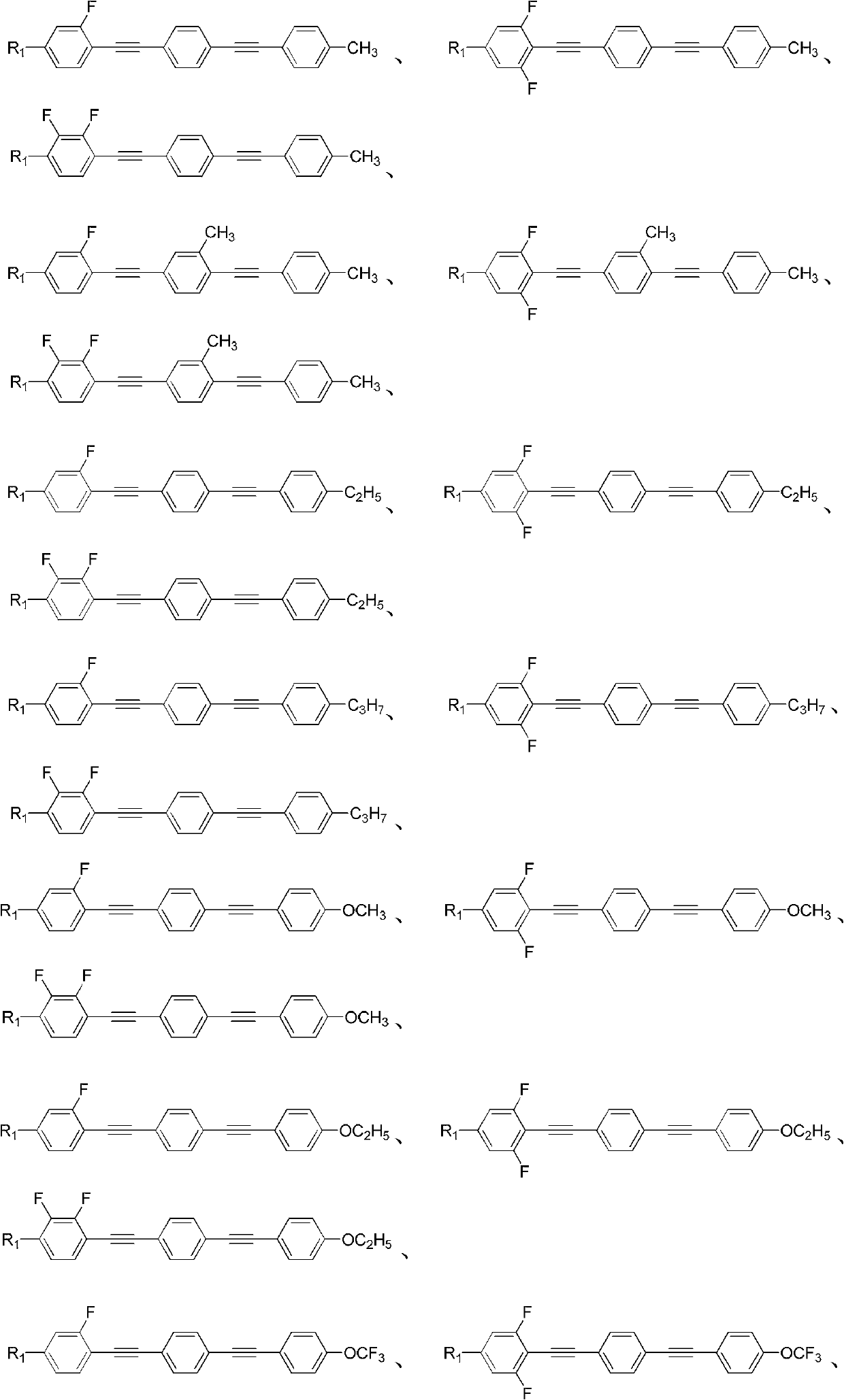
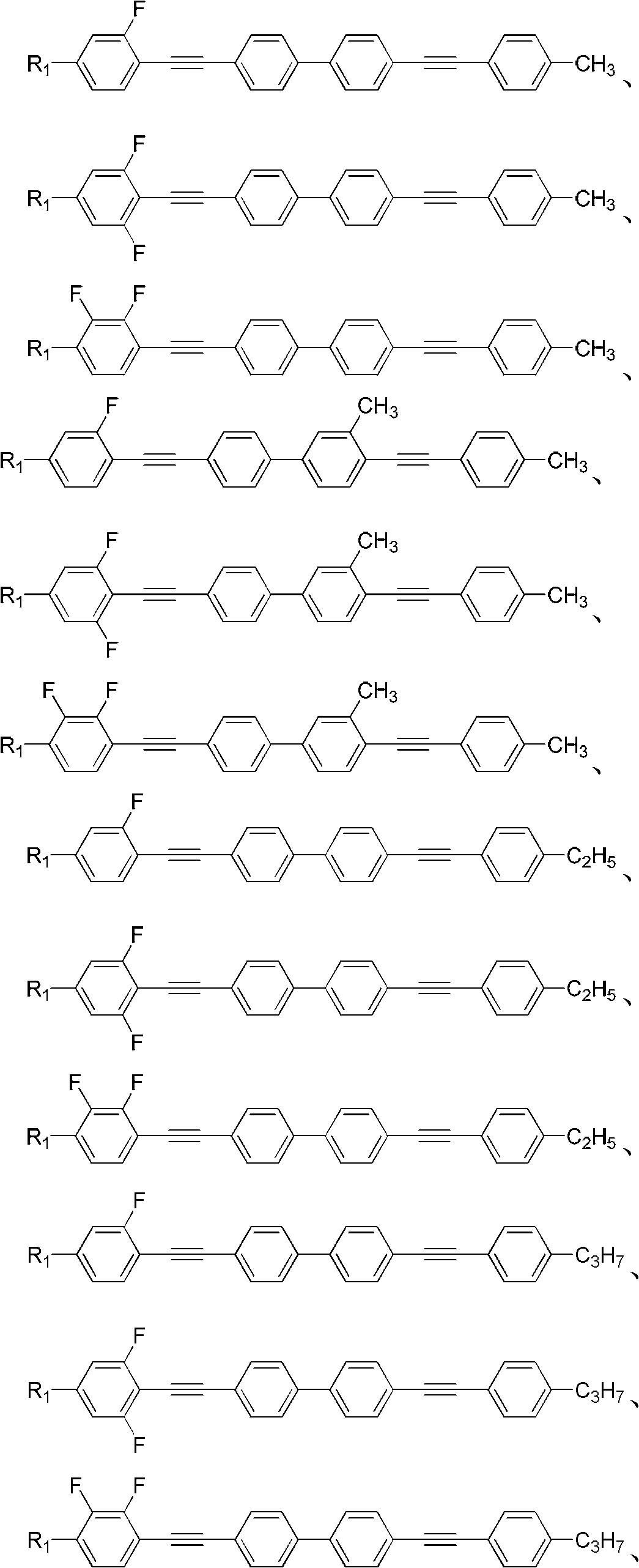
(Poly) fluorine substituted phenyl diacetylene (bi) phenyl derivative, and preparation method and application thereof
A technology of substituents and alkyl groups, used in the synthesis and application of liquid crystal compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] This example is the preparation of 2-((4-((4-ethoxyphenyl)ethynyl)phenyl)ethynyl)-1,3-difluoro-5-propylbenzene
[0054]
[0055] Step 1-1: Synthesis of the compound 4-(2,6-difluoro-4-propylphenyl)-2-methyl-3-butyn-2 alcohol shown in formula c
[0056]
[0057] Add 56.4 g of compound 1,3-difluoro-2-iodo-5-propylbenzene (0.2 mol) and 1 g of catalyst tetraphenylphosphine palladium (0.87 mmol, 0.4 mol%) in the three-necked flask With 50ml of tetrahydrofuran, the system was heated to 60°C under the protection of high-purity argon, and 21.8g (0.3mol) of 3-methyl-1-butyn-3-ol was added dropwise. After dropping, keep the temperature for coupling reaction for 5 hours, add saturated ammonium chloride aqueous solution to wash after cooling down, separate liquid extraction, separate through the column, and recrystallize from n-hexane to obtain the compound 4-(2,6-difluoro- 40.5 g (0.17 mol) of 4-propylphenyl)-2-methyl-3-butyn-2 alcohol, yield 85%.
[0058] Step 1-2: Synthes...
Embodiment 2
[0081] This example is the preparation of 5-ethyl-1,3-difluoro-2-((4-((4-methoxyphenyl)ethynyl)phenyl)ethynyl)benzene
[0082]
[0083] Step 1-1: Synthesis of 4-(4-ethyl-2,6-difluorophenyl)-2-methyl-3-butyn-2-ol
[0084]
[0085] Add 53.6 g of 5-ethyl-1,3-difluoro-2-iodobenzene (0.2 mol), 1 g of tetraphenylphosphine palladium (0.87 mmol, 0.4 mol%) and 50 ml of tetrahydrofuran into a three-necked flask, high-purity Under the protection of argon, the system was heated to 60°C, and 21.8 g (0.3 mol) of 3-methyl-1-butyn-3-ol was added dropwise. After dropping, keep the temperature for 5 hours, add saturated ammonium chloride aqueous solution to wash after cooling down, separate liquid extraction, separate through the column, and recrystallize from n-hexane to obtain the product 4-(4-ethyl-2,6-difluorophenyl)- 38.5 g (0.17 mol) of 2-methyl-3-butyn-2-ol.
[0086] Step 1-2: Synthesis of 5-ethyl-2-ethynyl-1,3-difluorobenzene
[0087]
[0088] 4-(4-Ethyl-2,6-difluorophenyl)-...
Embodiment 3
[0105] This example is the preparation of 4-((4-ethoxyphenyl)ethynyl)-4'-((2-fluoro-4-propylphenyl)ethynyl)biphenyl
[0106]
[0107] Step 1-1: Synthesis of 4-(2-fluoro-4-propylphenyl)-2-methyl-3-butyn-2-ol
[0108]
[0109] Add 52.8 g of 2-fluoro-1-iodo-4-propylbenzene (0.2 mol), 1 g of tetraphenylphosphine palladium (0.87 mmol, 0.4 mol%) and 50 ml of tetrahydrofuran into a three-necked flask, and protect the mixture with high-purity argon. The system was heated to 60° C., and 21.8 g (0.3 mol) of 3-methyl-1-butyn-3-ol was added dropwise. After dropping, keep the temperature for 5 hours, add saturated ammonium chloride aqueous solution to wash after cooling down, separate liquid extraction, separate through the column, and recrystallize from n-hexane to obtain the product 4-(2-fluoro-4-propylphenyl)-2-methanol 37.4 g (0.17 mol) of 3-butyn-2-ol.
[0110] Step 1-2: Synthesis of 1-ethynyl-2-fluoro-4-propylbenzene
[0111]
[0112] Put 4-(2-fluoro-4-propylphenyl)-2-met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com