Method for preparing sartan antihypertensive drug side-chain
An anti-hypertensive and sartan-like technology, which is applied in the field of preparation of pharmaceutical intermediates, can solve problems such as difficult purification and difficult control of bromination depth, and achieve the effects of less dibrominated by-products, high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
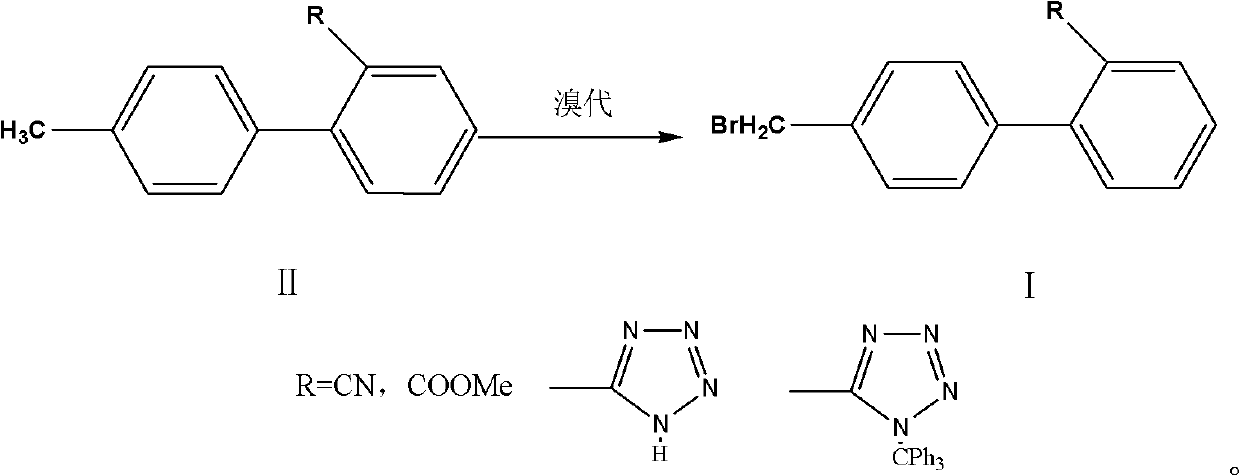
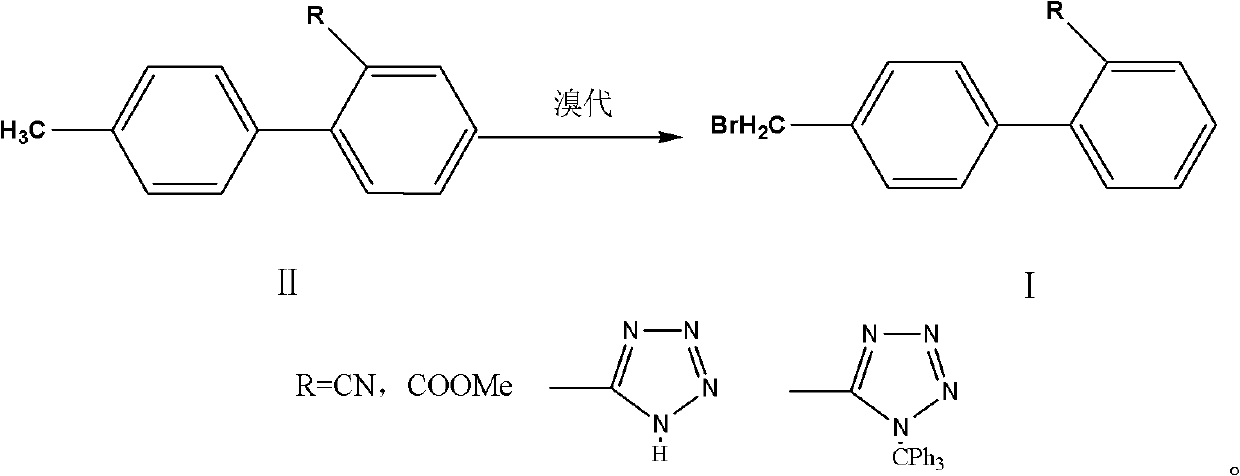
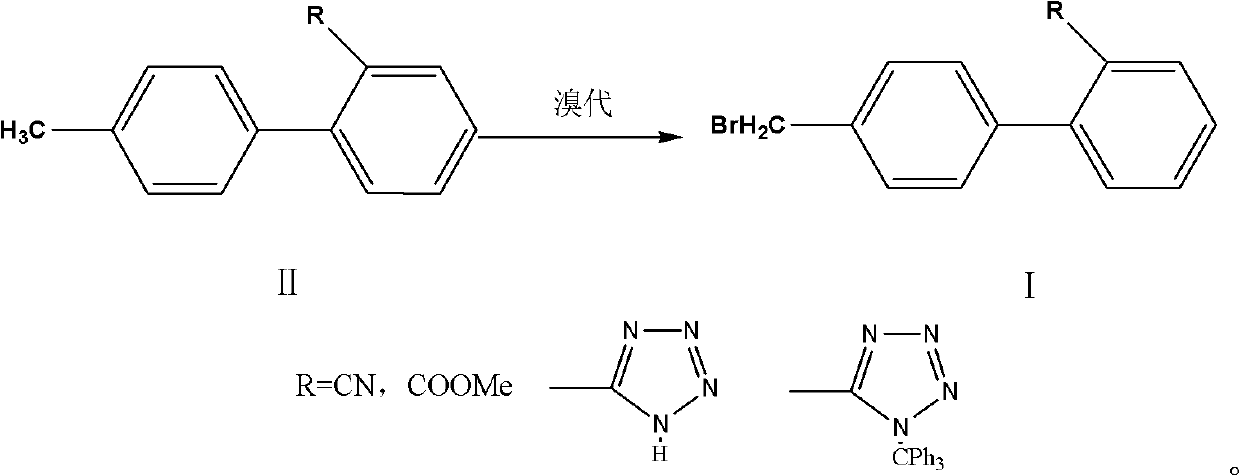
[0018] A preparation method for the side chain of sartan antihypertensive drugs, comprising: 4'-bromomethylbiphenyl-2-carbonitrile, 4'-bromomethylbiphenyl-2-carboxylate methyl ester, 5-[2 -(4'-Bromomethylbiphenyl)]-2-H-imidazole, 5-[2-(4'-Bromomethylbiphenyl)]-2-triphenylmethyl-imidazole are sartan drugs Synthetic, it is characterized in that: II (4'-methylbiphenyl-2-carbonitrile, 4'-methylbiphenyl-2-carboxylate methyl ester, 5-[2-(4'-methylbiphenyl) ]-2-H-imidazole, 5-[2-(4'-methylbiphenyl)]-2-triphenylmethyl-imidazole) dissolved in an aprotic organic solvent, adding 0.5 to 0.8 equivalents of brominated Then add the initiator, react for 1 to 6 hours, add the brominated agent and the initiator in stages, and add 0.02 to 0.2 equivalents of the brominated agent each time, until the basic reaction of the raw materials is complete, cool down to room temperature, wash and separate , reclaim the reaction solvent, add crystallization solvent in the raffinate and stir crystallization...
Embodiment I4
[0027] Embodiment 1 The synthesis of 4'-bromomethyl biphenyl-2-carbonitrile
[0028] In a 500ml flask, add 4'-methylbiphenyl-2-carbonitrile (38.7g, 0.2mol), 400ml of dichloromethane, and then dibromohydantoin (17.2g, 0.06mol), azobisisobutyronitrile 1.0g was heated up to reflux for 6 hours, cooled to 30-35°C, and dibromohydantoin and azobisisobutyronitrile were added in portions, adding dibromohydantoin (2.9g, 0.01mol) each time, azobis Isobutyronitrile 0.2g, after each reaction for 3 hours, stop the reaction until the HPLC of the raw material for phase detection is 96%, the dibromo product is <2%, and the raw material is < 1%.
Embodiment II4
[0029] Synthesis of embodiment II 4'-bromomethyl biphenyl-2-methyl carboxylate
[0030] In a 500ml flask, add 4'-methylbiphenyl-2-carboxylate methyl ester (45.3g, 0.2mol), ethyl acetate 400ml, add NBS (21.4g, 0.12mol), benzoyl peroxide 0.5 g, heat up to reflux for 6 hours, cool down to 62-67°C, add NBS and benzoyl peroxide in portions, add NBS (3.6g, 0.02mol) each time, benzoyl peroxide 0.2g, each time After reacting for 3 hours, stop the reaction until the HPLC of the phase detection material is 96%, dibromo products <2%, and raw materials <1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com